Left ventricular diastolic function compared to inferior vena cava diameter variation as predictor of fluid responsiveness in mechanical ventilated patients with shock: The research protocol
DOI:
https://doi.org/10.54205/ccc.v30.254873Keywords:
Fluid responsiveness, Shock, Left ventricular diastolic function, Mitral E/Ea ratio, Fluid challengeAbstract
Background: Fluid responsiveness, defined as an increase in cardiac output by 15% after a fluid challenge, is recommended to be evaluated in-patients with shock. Left ventricular (LV) diastolic dysfunction is associated with a lower increment of cardiac output after fluid challenge. Despite being a non-invasive test, the echocardiographic evaluation of the left ventricular diastolic function was rarely studied for the prediction of fluid responsiveness. The objective of this study is to evaluate the efficacy of LV diastolic function in predicting fluid responsiveness, comparing with inferior vena cava (IVC) diameter variation method, among shock patients who required mechanical ventilation.
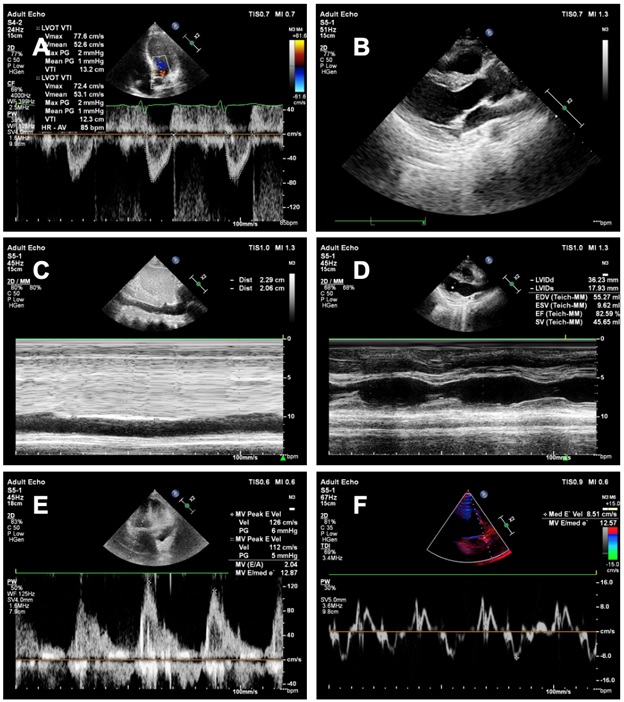
Methods: We plan to enroll adult patients with shock admitted to the intensive care unit (ICU). The echocardiographic hemodynamic parameters include IVC diameter variation, peak velocity of early diastolic filling of mitral valve inflow (E wave), peak early diastolic velocity of the mitral valve annulus (Ea), mitral E/Ea ratio, left ventricular ejection fraction (LVEF) and transaortic cardiac output (CO), all at baseline and after fluid therapy are measured. A fluid challenge with an infusion of 300 ml of acetate Ringer’s solution within 15 minutes will be given. Patients who have an increase in systolic blood pressure of at least 10 mmHg, mean arterial pressure of at least 5 mmHg or cardiac output of at least 15% are defined as fluid responders. The primary outcome of this study is the efficacy of the mitral E/Ea ratio comparing with IVC diameter variation in predicting fluid responsiveness. The secondary outcomes include the rate of fluid responsiveness in mechanically ventilated patients and LVEF and CO in patients with shock in the intensive care units.
Conclusion: This study will evaluate the efficacy of left ventricular diastolic function measured by the echocardiography (Mitral E/Ea ratio) in predicting fluid responsiveness among mechanical ventilated patients with shock.
Trial registrations: Clinicaltrials.gov NCT05066256, registered on January 10th, 2021
Downloads
References
Cecconi M, de Backer D, Antonelli M, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of In¬tensive Care Medicine. Intensive Care Medicine. 2014;40.
Monnet X, Teboul JL. Assessment of Fluid responsiveness. Hemodynamic Monitoring. ESICM 2019; 2019. p. 283–299.
Vincent J. Assessment of Adequacy of Cardiac Output. Hemodynamic Monitoring. ESICM 2019; 2019. p. 21–26.
Cecconi M, Hofer C, Teboul J-L, et al. Fluid challenges in intensive care: the FENICE study. Intensive Care Medicine. 2015;41.
Toscani L, Aya HD, Antonakaki D, et al. What is the impact of the fluid challenge technique on diagnosis of fluid responsiveness? A systematic review and meta-analysis. Critical Care. 2017;21.
Aya HD, Rhodes A, Chis Ster I, et al. Hemodynamic effect of different dos¬es of fluids for a fluid challenge: A quasi-randomized controlled study. Critical Care Medicine. 2017;45.
Mehta Y. Newer methods of cardiac output monitoring. World Journal of Cardiology. 2014;6.
Monnet X, Marik PE, Teboul JL. Prediction of fluid responsiveness: an up¬date. Annals of Intensive Care. 2016.
McLean AS, Needham A, Stewart D, et al. Estimation of cardiac output by noninvasive echocardiographic techniques in the critically ill subject. Anaesthesia and Intensive Care. 1997;25.
Mercado P, Maizel J, Beyls C, et al. Transthoracic echocardiography: An ac¬curate and precise method for estimating cardiac output in the critically ill patient. Critical Care. 2017;21.
Marques NR, de Riese J, Yelverton BC, et al. Diastolic Function and Periph¬eral Venous Pressure as Indices for Fluid Responsiveness in Cardiac Surgi¬cal Patients. Journal of Cardiothoracic and Vascular Anesthesia. 2019;33.
Tongyoo S, Thomrongpairoj P, Permpikul C. Efficacy of echocardiography during spontaneous breathing trial with low-level pressure support for predicting weaning failure among medical critically ill patients. Echocar¬diography. 2019;36.
Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the Eu¬ropean Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography. 2016;29.
Oxford Textbook of Advanced Critical Care Echocardiography. Oxford Textbook of Advanced Critical Care Echocardiography. 2020.
Tongyoo S, Sangnopakunsri W, Permpikul C. Transthoracic echocardio¬gram for the diagnosis of right ventricular dysfunction in critically Ill pa¬tients. Journal of the Medical Association of Thailand. 2014;97.
Quintard H, Muller L, Philip I, et al. Influence of acute preload changes on mitral annulus velocity measured by tissue Doppler echocardiography in critically ill patients. Journal of Clinical Ultrasound. 2012;40.
Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: inter¬national guidelines for management of sepsis and septic shock 2021. Intensive Care Medicine. 2021;47.
Semler MW, Self WH, Wanderer JP, et al. Balanced Crystalloids versus Sa¬line in Critically Ill Adults. New England Journal of Medicine. 2018;378.
Yunos NRAM, Bellomo R, Hegarty FC, et al. Association between a chlo¬ride-liberal vs chloride-restrictive intravenous fluid administration strat¬egy and kidney injury in critically ill adults. JAMA - Journal of the Ameri¬can Medical Association. 2012;308.
Malbrain MLNG, van Regenmortel N, Saugel B, et al. Principles of fluid management and stewardship in septic shock: it is time to consider the four D’s and the four phases of fluid therapy. Annals of Intensive Care. 2018.
Hahn RG. Volume kinetics for infusion fluids. Anesthesiology. 2010.
Hahn RG, Lyons G. The half-life of infusion fluids. European Journal of Anaesthesiology. 2016.
Pierrakos C, Velissaris D, Scolletta S, et al. Can changes in arterial pressure be used to detect changes in cardiac index during fluid challenge in pa¬tients with septic shock? Intensive Care Medicine. 2012.
Monnet X, Letierce A, Hamzaoui O, et al. Arterial pressure allows moni¬toring the changes in cardiac output induced by volume expansion but not by norepinephrine. Critical Care Medicine. 2011;39.
García MIM, Romero MG, Cano AG, et al. Dynamic arterial elastance as a predictor of arterial pressure response to fluid administration: A valida¬tion study. Critical Care. 2014;18.
Muller L, Toumi M, Bousquet PJ, et al. An increase in aortic blood flow after an infusion of 100 ml colloid over 1 minute can predict fluid respon¬siveness: The mini-fluid challenge study. Anesthesiology. 2011;115.
Huang H, Shen Q, Liu Y, et al. Value of variation index of inferior vena cava diameter in predicting fluid responsiveness in patients with circu-latory shock receiving mechanical ventilation: A systematic review and meta-analysis. Critical Care. 2018;22.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 The Thai Society of Critical Care Medicine

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.




