Continuous vancomycin infusion versus intermittent infusion in critically Ill patients: The research protocol
DOI:
https://doi.org/10.54205/ccc.v30.254939Keywords:
Continuous infusion, Pharmacodynamic, VancomycinAbstract
Background: Methicillin‐resistant Staphylococcal and Enterococcal infections are important problems in intensive care units (ICUs). Vancomycin is a drug of choice, and continuous administration has long been proposed as an alternative method with better therapeutic benefits. This study aims to examine information on the benefits of continuous vancomycin infusion (CVI) compared with the intermittent vancomycin infusion (IVI) method.
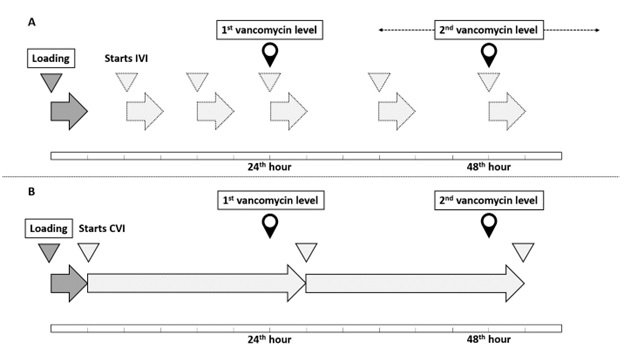
Method: A quasi-experimental study with a propensity score-matched historical control involves adult patients in medical or surgical ICUs. In the experimental group, 31 patients for whom vancomycin is indicated will be enrolled to receive CVI for at least 48 hours with therapeutic drug monitoring according to the study protocol. For the historical control group, data of patients who received IVI between January 2018 and October 2020 will be retrospectively reviewed. Capability to achieve serum vancomycin therapeutic target within 48 hours, 96 hours, the incidence of supra- and subtherapeutic level, treatment successfulness, mortality, and incidence of acute kidney injury (AKI) between the two infusion methods will be analyzed before and after one-to-two propensity score matching.
Ethics and dissemination: The study was approved by the institutional review boards of Faculty of Medicine Siriraj Hospital, Mahidol University (COA no. Si 027/2021). We plan to disseminate the results in peer-reviewed critical care medicine or infectious disease-related journals and national and international conferences.
Trial registration: TCTR20210122005. Registered on January 22, 2021, with Thai Clinical Trials Registry
Downloads
References
Zamoner W, Prado IRS, Balbi AL, Ponce D. Vancomycin dosing, monitoring and toxicity: Critical review of the clinical practice. Clin Exp Pharmacol Physiol. 2019. Epub 2019/01/10. doi: 10.1111/1440-1681.13066. PubMed PMID: 30623980.
Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clinical Infectious Diseases. 2006;42(Supplement_1):S35-S9.
DiMondi VP, Rafferty K. Review of continuous-infusion vancomycin. Ann Pharmacother. 2013;47(2):219-27. Epub 2013/02/07. doi: 10.1345/aph.1R420. PubMed PMID: 23386074.
Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clinical pharmacokinetics. 2004;43(13):925-42.
Song KH, Kim HB, Kim HS, Lee MJ, Jung Y, Kim G, et al. Impact of area under the concentration-time curve to minimum inhibitory concentration ratio on vancomycin treatment outcomes in methicillin-resistant Staphylococcus aureus bacteraemia. Int J Antimicrob Agents. 2015;46(6):689-95. Epub 2015/11/12. doi: 10.1016/j.ijantimicag.2015.09.010. PubMed PMID: 26555059.
Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC, Craig WA, Billeter M, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49(3):325-7. Epub 2009/07/03. doi: 10.1086/600877. PubMed PMID: 19569969.
Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-64. Epub 2020/03/20. doi: 10.1093/ajhp/zxaa036. PubMed PMID: 32191793.
Meng L, Fang Y, Chen Y, Zhu H, Long R. High versus low vancomycin serum trough regimen for Gram-positive infections: a meta-analysis. Journal of Chemotherapy. 2015;27(4):213-20.
Nakakura I, Sakakura K, Imanishi K, Sako R, Yamazaki K. Association between vancomycin pharmacokinetic/pharmacodynamic parameters, patient characteristics, and mortality in patients with bacteremia caused by vancomycin-susceptible Enterococcus faecium: a single-center retrospective study. Journal of Pharmaceutical Health Care and Sciences. 2019;5(1):1-8.
Duszynska W, Taccone FS, Hurkacz M, Wiela-Hojenska A, Kübler A. Continuous vs. intermittent vancomycin therapy for Gram-positive infections not caused by methicillin-resistant Staphylococcus aureus. Minerva anestesiologica. 2015;82(3):284-93.
Wysocki M, Delatour F, Faurisson F, Rauss A, Pean Y, Misset B, et al. Continuous versus intermittent infusion of vancomycin in severe Staphylococcal infections: prospective multicenter randomized study. Antimicrob Agents Chemother. 2001;45(9):2460-7. Epub 2001/08/15. doi: 10.1128/aac.45.9.2460-2467.2001. PubMed PMID: 11502515; PubMed Central PMCID: PMCPMC90678.
Schmelzer TM, Christmas AB, Norton HJ, Heniford BT, Sing RF. Vancomycin intermittent dosing versus continuous infusion for treatment of ventilator-associated pneumonia in trauma patients. The American Surgeon. 2013;79(11):1185-90.
Hong LT, Goolsby TA, Sherman DS, Mueller SW, Reynolds P, Cava L, et al. Continuous infusion vs intermittent vancomycin in neurosurgical intensive care unit patients. J Crit Care. 2015;30(5):1153 e1-6. Epub 2015/08/05. doi: 10.1016/j.jcrc.2015.06.012. PubMed PMID: 26239323.
Bissell BD, Riggi G, Morrison C. Evaluation of Continuous Infusion Vancomycin Administration in a Critically Ill Trauma Population. J Intensive Care Med. 2020;35(6):570-5. Epub 2018/04/13. doi: 10.1177/0885066618768749. PubMed PMID: 29642744.
Flannery AH, Bissell BD, Bastin MT, Morris PE, Neyra JA. Continuous Versus Intermittent Infusion of Vancomycin and the Risk of Acute Kidney Injury in Critically Ill Adults: A Systematic Review and Meta-Analysis. Crit Care Med. 2020;48(6):912-8. Epub 2020/04/23. doi: 10.1097/CCM.0000000000004326. PubMed PMID: 32317590.
Hao JJ, Chen H, Zhou JX. Continuous versus intermittent infusion of vancomycin in adult patients: A systematic review and meta-analysis. Int J Antimicrob Agents. 2016;47(1):28-35. Epub 2015/12/15. doi: 10.1016/j.ijantimicag.2015.10.019. PubMed PMID: 26655032.
Raverdy V, Ampe E, Hecq J-D, Tulkens PM. Stability and compatibility of vancomycin for administration by continuous infusion. Journal of Antimicrobial Chemotherapy. 2013;68(5):1179-82.
Cockcroft DW, Gault H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31-41.
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. Epub 2007/03/03. doi: 10.1186/cc5713. PubMed PMID: 17331245; PubMed Central PMCID: PMCPMC2206446.
Waineo MF, Kuhn TC, Brown DL. The pharmacokinetic/pharmacodynamic rationale for administering vancomycin via continuous infusion. Journal of Clinical Pharmacy and Therapeutics. 2015;40(3):259-65. doi: 10.1111/jcpt.12270.
Tafelski S, Nachtigall I, Troeger U, Deja M, Krannich A, Gunzel K, et al. Observational clinical study on the effects of different dosing regimens on vancomycin target levels in critically ill patients: Continuous versus intermittent application. J Infect Public Health. 2015;8(4):355-63. Epub 2015/03/22. doi: 10.1016/j.jiph.2015.01.011. PubMed PMID: 25794497.
Hutschala D, Kinstner C, Skhirdladze K, Thalhammer F, Müller M, Tschernko E. Influence of Vancomycin on Renal Function in Critically Ill Patients after Cardiac Surgery: Continuous versusIntermittent Infusion. The Journal of the American Society of Anesthesiologists. 2009;111(2):356-65.
Saugel B, Nowack MC, Hapfelmeier A, Umgelter A, Schultheiss C, Thies P, et al. Continuous intravenous administration of vancomycin in medical intensive care unit patients. J Crit Care. 2013;28(1):9-13. Epub 2012/03/31. doi: 10.1016/j.jcrc.2012.02.003. PubMed PMID: 22459156.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 The Thai Society of Critical Care Medicine

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.




