Terlipressin for refractory septic shock: a study protocol of a single center, placebo-controlled double-blind phase III RCT (The TERESEP study).
DOI:
https://doi.org/10.54205/ccc.v30.254966Keywords:
Terlipressin, Septic shock, Catecholamine, Randomized controlled clinical trialAbstract
Introduction: In septic shock, vasopressin is a standard treatment that increases blood pressure by vasopressin receptor activation. Vasopressin can reduce catecholamine dose requirement and reduce cardiac arrhythmia in septic shock. Terlipressin is specific vasopressin 1 receptor that may replace vasopressin for septic shock treatment.The TERESEP trial evaluates the benefit of terlipressin add-on catecholamine versus catecholamine only treatment for septic shock.
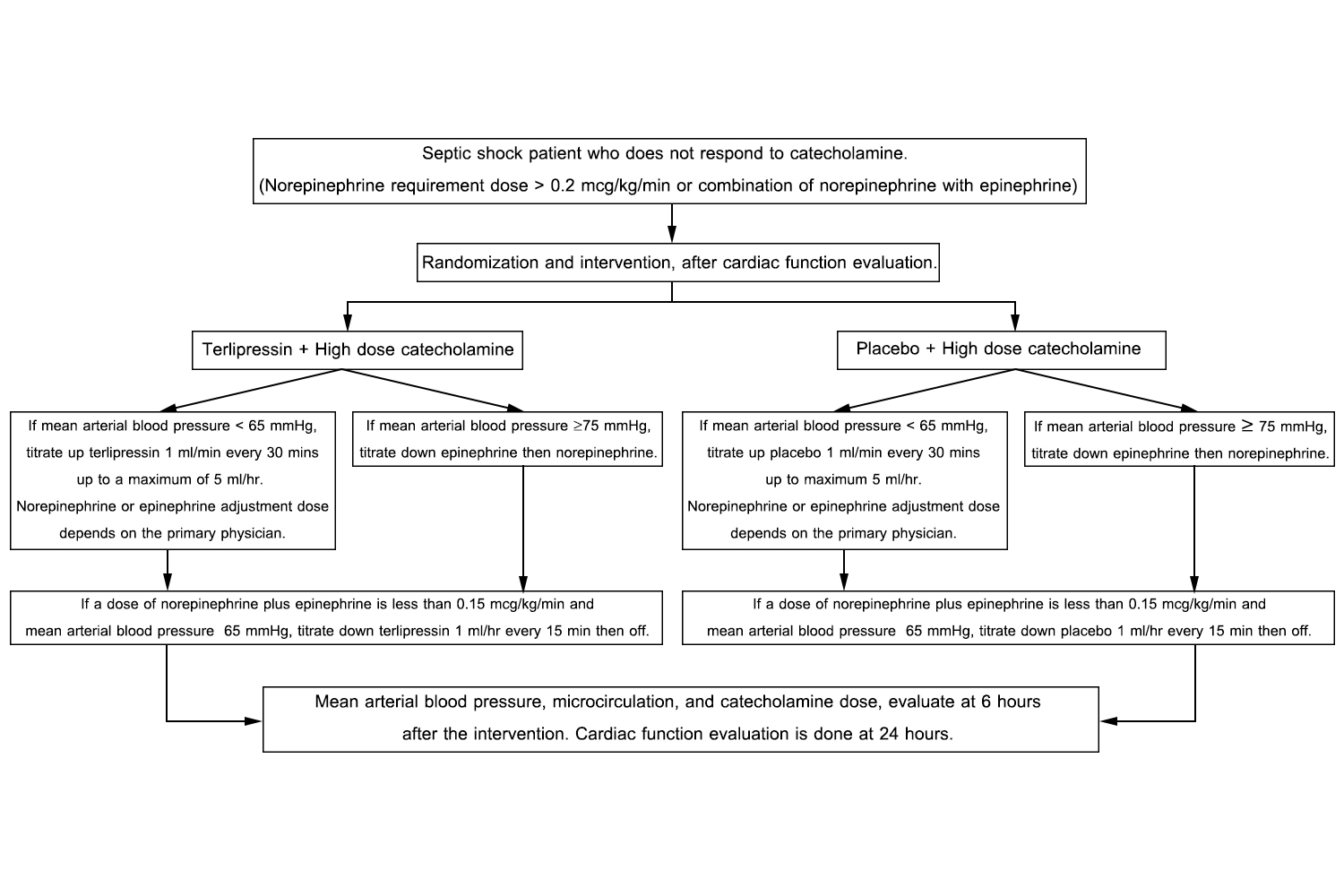
Methods and analysis: This single-center randomized controlled clinical trial is enrolling hospitalized intensive care patients with septic shock with norepinephrine doses of more than 0.2 microgram/kilogram/min or norepinephrine combine with epinephrine. Patient randomized for terlipressin combined with catecholamine or placebo combined with catecholamine. The primary endpoint is successful of shock treatment within 6 hours define as the rate of mean arterial blood pressure more than 65 mmHg achievement with catecholamine requirement dose less than 0.2 mcg/kg/min. The secondary outcomes include mean blood pressure, 28 days mortality, hospital mortality, intensive care unit range of stay, rate of urine output achievement, lactate clearance, accumulative catecholamine dose, cardiac arrhythmia, 28 days alive without any organ support. The main analysis will use intension to treat approach.
Ethic and dissemination: The Ethics Committee has approved this study of Siriraj hospital, Mahidol University (COA No. SI 049/2020). The trial result will be disseminated through the presentation at medical publication. Authorship will consider and grant using the policy of Mahidol University.
Trial registrations: ClinicalTrials.govNCT04339868. Registered on April 9,2020.
Downloads
References
Vincent J-L, De Backer D. Circulatory Shock. New England Journal of Medicine 2013;369:1726-34.
Vincent J-L, Ince C, Bakker J. Clinical review: Circulatory shock - an update: a tribute to Professor Max Harry Weil. Critical Care 2012;16:239.
De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, et al. Comparison of Dopamine and Norepinephrine in the Treatment of Shock. New England Journal of Medicine 2010;362:779-89.
Angkasekwinai N, Rattanaumpawan P, Thamlikitkul V. Epidemiology of sepsis in Siriraj Hospital 2007. J Med Assoc Thai 2009;92 Suppl 2:S68-78.
Fleischmann-Struzek C, Mellhammar L, Rose N, Cassini A, Rudd KE, Schlattmann P, et al. Incidence and mortality of hospital- and ICU-treated sepsis: results from an updated and expanded systematic review and meta-analysis. Intensive Care Medicine 2020;46:1552-62.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Medicine 2021. http://dx.doi.org/10.1007/s00134-021-06506-y.
Holmes CL, Patel BM, Russell JA, Walley KR. Physiology of vasopressin relevant to management of septic shock. Chest 2001;120:989-1002.
Landry DW, Levin HR, Gallant EM, Ashton RC, Jr., Seo S, D’Alessandro D, et al. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation 1997;95:1122-5.
Russell JA, Walley KR, Singer J, Gordon AC, Hébert PC, Cooper DJ, et al. Vasopressin versus Norepinephrine Infusion in Patients with Septic Shock. New England Journal of Medicine 2008;358:877-87.
Gordon AC, Russell JA, Walley KR, Singer J, Ayers D, Storms MM, et al. The effects of vasopressin on acute kidney injury in septic shock. Intensive Care Med 2010;36:83-91.
Gordon AC, Mason AJ, Thirunavukkarasu N, Perkins GD, Cecconi M, Cepkova M, et al. Effect of Early Vasopressin vs Norepinephrine on Kidney Failure in Patients With Septic Shock: The VANISH Randomized Clinical Trial. JAMA 2016;316:509-18.
Nagendran M, Russell JA, Walley KR, Brett SJ, Perkins GD, Hajjar L, et al. Vasopressin in septic shock: an individual patient data meta-analysis of randomised controlled trials. Intensive Care Med 2019;45:844-55.
Morelli A, Ertmer C, Rehberg S, Lange M, Orecchioni A, Cecchini V, et al. Continuous terlipressin versus vasopressin infusion in septic shock (TERLIVAP): a randomized, controlled pilot study. Crit Care 2009;13:R130.
Liu ZM, Chen J, Kou Q, Lin Q, Huang X, Tang Z, et al. Terlipressin versus norepinephrine as infusion in patients with septic shock: a multicentre, randomised, double-blinded trial. Intensive Care Med 2018;44:1816-25.
Zhu Y, Huang H, Xi X, Du B. Terlipressin for septic shock patients: a meta-analysis of randomized controlled study. Journal of Intensive Care 2019;7:16.
Serpa Neto A, Nassar AP, Cardoso SO, Manetta JA, Pereira VG, Espósito DC, et al. Vasopressin and terlipressin in adult vasodilatory shock: a systematic review and meta-analysis of nine randomized controlled trials. Crit Care 2012;16:R154.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10.
Khanna A, English SW, Wang XS, Ham K, Tumlin J, Szerlip H, et al. Angiotensin II for the Treatment of Vasodilatory Shock. New England Journal of Medicine 2017;377:419-30.
Yamazaki Y, Oba K, Matsui Y, Morimoto Y. Vasoactive-inotropic score as a predictor of morbidity and mortality in adults after cardiac surgery with cardiopulmonary bypass. J Anesth 2018;32:167-73.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 The Thai Society of Critical Care Medicine

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.




