Clinical efficacy of hemoperfusion with a cytokine adsorbent in norepinephrine-resistant septic shock: protocol for the CLEANSE randomized clinical trial
Protocol for the CLEANSE randomized clinical trial
DOI:
https://doi.org/10.54205/ccc.v30.255033Keywords:
Blood purification, Cytokine, HA-330, Hemoperfusion, Sepsis, Septic shockAbstract
Background: Due to the pivotal role of inflammatory cytokines in sepsis, hemoperfusion with cytokine adsorbents may lead to better outcomes. Although previous studies showed inconclusive results, proper patient selection and timing of hemoperfusion may lead to improved survival.
Objectives: To examine whether patients with septic shock requiring high-dose vasopressors undergoing add-on hemoperfusion with a cytokine adsorbent have better clinical outcomes than those treated with standard treatment alone.
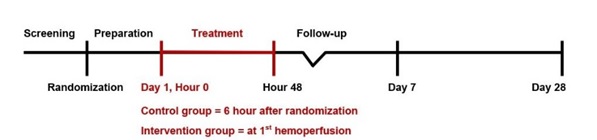
Methods: This is a multi-center, randomized controlled study in 2 tertiary care centers. 206 patients with septic shock receiving norepinephrine of 0.2 mcg/kg/min or higher are randomized to receive either standard treatment combined with 3-hour sessions of hemoperfusion with cytokine adsorbent for two consecutive days (HP group) or standard treatment alone (ST group). The primary outcome is 28-day mortality. Secondary outcomes include hospital and ICU mortality, shock reversal, vasoactive-inotropic score (VIS), organ support-free days, interleukin-6 levels, as well as safety data.
Conclusions: This study will provide information to guide the use of hemoperfusion with a cytokine adsorbent in patients with septic shock.
Downloads
References
Vincent JL, Jones G, David S, Olariu E, Cadwell KK. Frequency and mortality of septic shock in Europe and North America: a systematic review and meta-analysis. Crit Care. 2019;23(1):196.
Khanna A, Ostermann M, Bellomo R. Angiotensin II for the Treatment of Vasodilatory Shock. N Engl J Med. 2017;377(26):2604.
Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810.
Rice TW. Treatment of severe sepsis: where next? Current and future treatment approaches after the introduction of drotrecogin alfa. Vasc Health Risk Manag. 2006;2(1):3-18.
Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181-1247.
Ankawi G, Fan W, Pomarè Montin D, et al. A New Series of Sorbent Devices for Multiple Clinical Purposes: Current Evidence and Future Directions. Blood Purif. 2019;47(1-3):94-100.
Huang Z, Wang SR, Su W, Liu JY. Removal of humoral mediators and the effect on the survival of septic patients by hemoperfusion with neutral microporous resin column. Ther Apher Dial. 2010;14(6):596-602.
Bozza FA, Salluh JI, Japiassu AM, et al. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007;11(2):R49.
Belletti A, Lerose CC, Zangrillo A, Landoni G. Vasoactive-Inotropic Score: Evolution, Clinical Utility, and Pitfalls. J Cardiothorac Vasc Anesth. 2021;35(10):3067-3077.
Khanna A, English SW, Wang XS, et al. Angiotensin II for the Treatment of Vasodilatory Shock. N Engl J Med. 2017;377(5):419-430.
Yehya N, Harhay MO, Curley MAQ, Schoenfeld DA, Reeder RW. Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med. 2019;200:828–36.
Honore PM, Hoste E, Molnár Z, et al. Cytokine removal in human septic shock: Where are we and where are we going?. Ann Intensive Care. 2019;9(1):56.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 The Thai Society of Critical Care Medicine

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.




