Rational use of corticosteroid treatment in the early phase of severe COVID-19
Corticosteroid in COVID-19
DOI:
https://doi.org/10.54205/ccc.v31.259411Keywords:
COVID-19, SARS-CoV-2, Corticosteroid, Critical ill, ImmunomodulatorAbstract
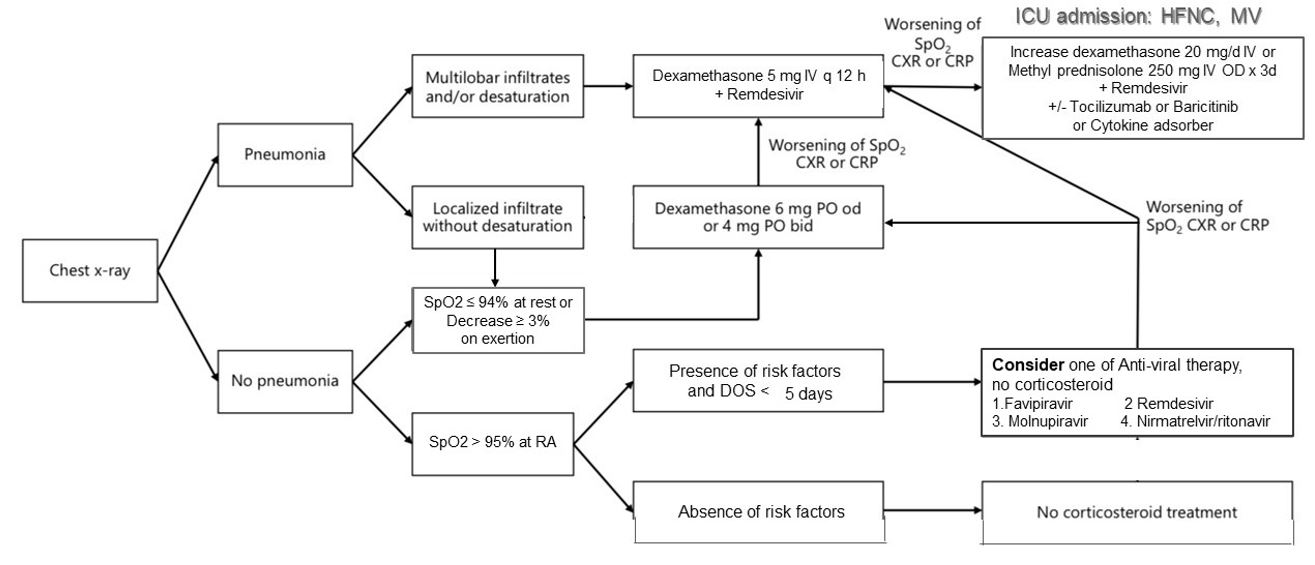
Mechanisms of hypoxemia in COVID-19 pneumonia include pulmonary inflammation, alveolar collapse, atelectasis, and pulmonary intravascular coagulopathy due to a hyperinflammatory response to SARS-CoV-2 infection. Systemic corticosteroids are widely applied as a standard treatment for hospitalized COVID-19 patients after several studies have shown favorable outcomes. However, the standard dosing and tailoring of corticosteroids in COVID-19 patients have not been established. Differences in dosing and timing of corticosteroid use may affect the outcome of COVID-19 patients. Inappropriate use of corticosteroids can lead to less benefit and potentially harmful adverse events. Dexamethasone is the most widely used corticosteroid as a result of the positive outcome from the RECOVERY study and its high anti-inflammatory potency. Although several studies have shown the benefit of higher dose corticosteroids in severe COVID-19 patients, serious adverse events associated with the use of corticosteroids, such as superimposed bacterial and/or fungal infections, have also been observed. Therefore, in this article, we reviewed current evidence of corticosteroid usage in COVID-19 patients and suggested a strategy for tailoring corticosteroid usage according to the clinical severity and risk of the patients.
Downloads
References
Ratanarat R, Sivakorn C, Viarasilpa T, et al. Critical care management of patients with COVID-19: Early experience in Thailand. Amer J Trop Med Hygiene. 2020;103(1):48–54.
Dexamethasone in Hospitalized Patients with Covid-19. New England J Medicine [Internet]. 2021;384:693–704. Available from: http://www.nejm.org/doi/10.1056/NEJMoa2021436.
COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health.
Sterne JAC, Murthy S, Diaz J v., et al. Association between Administration of Systemic Corticosteroids and Mortality among Critically Ill Patients with COVID-19: A Meta-analysis. JAMA. 2020;324(13):1330-1341.
Osuchowski MF, Winkler MS, Skirecki T, et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir Med. 2021;9(6):622-642.
McGonagle D, Bridgewood C, Meaney JFM. A tricompartmental model of lung oxygenation disruption to explain pulmonary and systemic pathology in severe COVID-19. Lancet Respir Med. 2021;9(6):665-672.
Yu M, Liu Y, Xu D, et al. Prediction of the development of pulmonary fibrosis using serial thin-section ct and clinical features in patients discharged after treatment for COVID-19 pneumonia. Korean J Radiol. 2020;21(6):746-755.
Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
Balnis J, Adam AP, Chopra A, et al. Unique inflammatory profile is associated with higher SARS-CoV-2 acute respiratory distress syndrome (ARDS) mortality. Am J Physiol Regul Integr Comp Physiol. 2021;320(3):R250-R257.
Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846-848.
Kox M, Waalders NJB, Kooistra EJ, et al. Cytokine Levels in Critically Ill Patients with COVID-19 and Other Conditions. JAMA. 2020;324(15):1565-1567.
Sinha P, Matthay MA, Calfee CS. Is a “cytokine Storm” Relevant to COVID-19? JAMA Intern Med. 2020;180(9):1152-1154.
Hojyo S, Uchida M, Tanaka K, et al. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 2020;40:37.
Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405-407.
Tongyoo S, Permpikul C, Mongkolpun W, et al. Hydrocortisone treatment in early sepsis-associated acute respiratory distress syndrome: Results of a randomized controlled trial. Crit Care. 2016;20(1):329.
Villar J, Ferrando C, Martínez D, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8(3):267-276.
Chaudhuri D, Sasaki K, Karkar A, et al. Corticosteroids in COVID-19 and non-COVID-19 ARDS: a systematic review and meta-analysis. Intensive Care Med. 2021;47(5):521-537.
Williams DM. Clinical pharmacology of corticosteroids. Respir Care. 2018.63(6):655-670.
Rhen T, Cidlowski JA. Antiinflammatory Action of Glucocorticoids — New Mechanisms for Old Drugs. New England J Medicine. 2005;353(16):1711-23.
Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients with Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA. 2020;324(13):1307-1316.
Dequin PF, Heming N, Meziani F, et al. Effect of Hydrocortisone on 21-Day Mortality or Respiratory Support among Critically Ill Patients with COVID-19: A Randomized Clinical Trial. JAMA. 2020;324(13):1298-1306.
Angus DC, Derde L, Al-Beidh F, et al. Effect of Hydrocortisone on Mortality and Organ Support in Patients with Severe COVID-19: The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial. JAMA. 2020;324(13):1317-1329.
Jeronimo CMP, Farias MEL, Val FFA, et al. Methylprednisolone as Adjunctive Therapy for Patients Hospitalized With Coronavirus Disease 2019 (COVID-19; Metcovid): A Randomized, Double-blind, Phase IIb, Placebo-controlled Trial. Clin Infect Dis. 2021;72(9):e373-e381.
Edalatifard M, Akhtari M, Salehi M, et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: Results from a randomised controlled clinical trial. Eur Respir J. 2020;56(6):2002808.
Munch MW, Myatra SN, Vijayaraghavan BKT, et al. Effect of 12 mg vs 6 mg of Dexamethasone on the Number of Days Alive without Life Support in Adults with COVID-19 and Severe Hypoxemia: The COVID STEROID 2 Randomized Trial. JAMA. 2021;326(18):1807-1817.
Vichyanond P, Irvin CG, Larsen GL, et al. Penetration of corticosteroids into the lung: Evidence for a difference between methylprednisolone and prednisolone. J Allergy Clin Immunol. 1989;84:867-73.
Ranjbar K, Moghadami M, Mirahmadizadeh A, et al. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. BMC Infect Dis. 2021;21(1):337.
Pinzón MA, Ortiz S, Holguín H, et al. Dexamethasone vs methylprednisolone high dose for Covid-19 pneumonia. PLoS One. 2021;16(5):e0252057.
Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181-1247.
Munch MW, Meyhoff TS, Helleberg M, et al. Low-dose hydrocortisone in patients with COVID-19 and severe hypoxia: The COVID STEROID randomised, placebo-controlled trial. Acta Anaesthesiol Scand. 2021;65(10):1421-1430.
Ramakrishnan S, Nicolau D v., Langford B, et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med. 2021;9(7):763-772.
Yu LM, Bafadhel M, Dorward J, et al. Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;398(10303):843-855.
Granholm A, Munch MW, Myatra SN, et al. Dexamethasone 12 mg versus 6 mg for patients with COVID-19 and severe hypoxaemia: a pre-planned, secondary Bayesian analysis of the COVID STEROID 2 trial. Intensive Care Med. 2021;48(1):45-55.
Corral-Gudino L, Bahamonde A, Arnaiz-Revillas F, et al. GLUCOCOVID: A controlled trial of methylprednisolone in adults hospitalized with COVID-19 pneumonia. Wien Klin Wochenschr. 2020;133(7-8):303-311.
Angriman F, Ferreyro BL, Burry L, et al. Interleukin-6 receptor blockade in patients with COVID-19: placing clinical trials into context. Lancet Respir Med. 2021;9(6):655-664.
Tang X, Feng YM, Ni JX, et al. Early Use of Corticosteroid May Prolong SARS-CoV-2 Shedding in Non-Intensive Care Unit Patients with COVID-19 Pneumonia: A Multicenter, Single-Blind, Randomized Control Trial. Respiration. 2021;100(2):116-126.
Li TZ, Cao ZH, Chen Y, et al. Duration of SARS-CoV-2 RNA shedding and factors associated with prolonged viral shedding in patients with COVID-19. J Med Virol. 2021;93(1):506-512.
Fekkar A, Lampros A, Mayaux J, et al. Occurrence of invasive pulmonary fungal infections in patients with severe COVID-19 admitted to the ICU. Am J Respir Crit Care Med. 2021;203(3):307-317.
Al-Tawfiq JA, Alhumaid S, Alshukairi AN, et al. COVID-19 and mucormycosis superinfection: the perfect storm. Infection. 2021;49(5):833-853.
Giacobbe DR, Battaglini D, Ball L, et al. Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest. 2020;50(10):e13319.
Khunti K, del Prato S, Mathieu C, et al. COVID-19, Hyperglycemia, and New-Onset Diabetes. Diabetes Care. 2021;44(12):2645-2655.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 The Thai Society of Critical Care Medicine

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.




