Composite adverse events compared early versus conventional cessation of hydrocortisone in patients with septic shock: Randomized-controlled trial (The CESSHYDRO study)
Cessation of hydrocortisone in septic shock patient
DOI:
https://doi.org/10.54205/ccc.v32.266229Keywords:
Hydrocortisone, Septic shock, Sepsis with hypotension, hyperglycemia, VasopressorAbstract
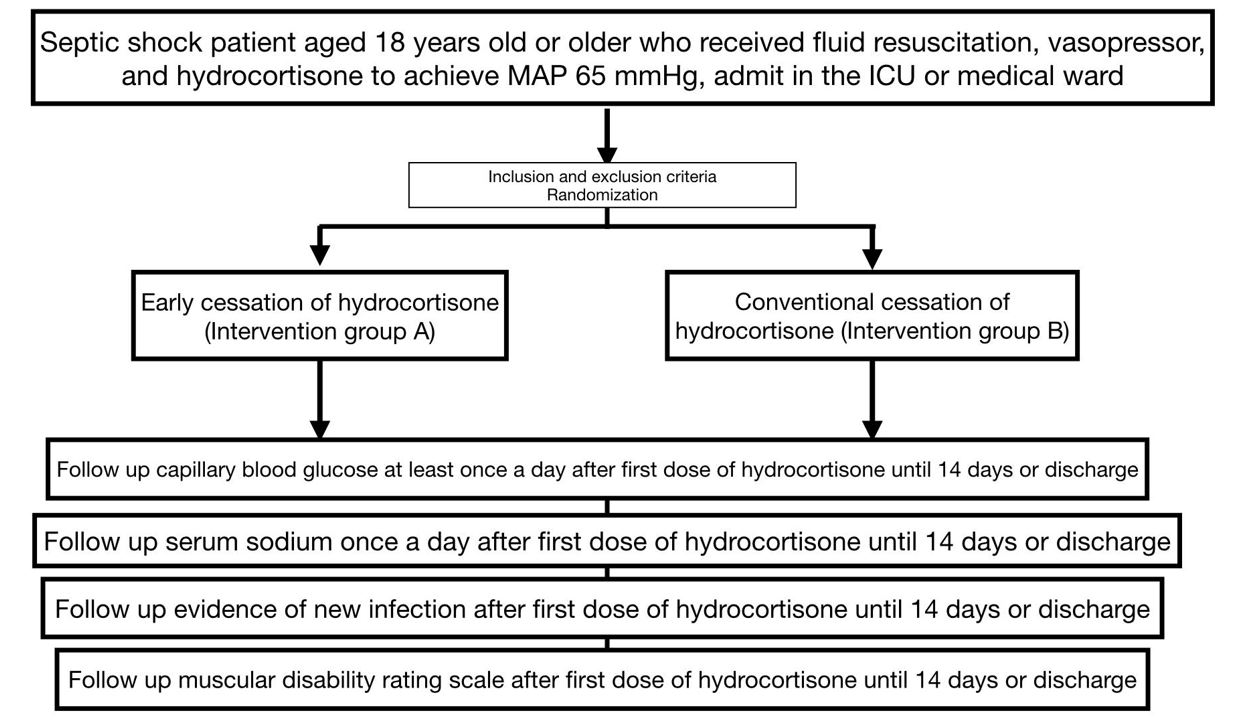
Background: Intravenous hydrocortisone has benefits in the treatment of septic shock patients, but there are adverse events mentioned in the secondary outcomes of several studies, such as hyperglycemia, hypernatremia, secondary infection, and muscle weakness. In addition, there are no recommendations regarding the precise duration and steps to discontinue hydrocortisone administration. The CESSHYDRO trial evaluates the adverse outcomes of intravenous hydrocortisone between early cessation versus conventional cessation of hydrocortisone in septic shock patients.
Methods: The CESSHYDRO trial is a single-center, double-blind, randomized controlled clinical trial conducted at Siriraj Hospital. One hundred and eighty septic shock patients receiving vasopressors and hydrocortisone at least 200 mg/day with hemodynamic stability will be included. The patients are randomized into 2 groups: intervention A (early cessation of hydrocortisone) and intervention B (conventional cessation). The primary outcomes were composite adverse events, including hyperglycemia, hypernatremia, muscle weakness, and new infections.
Hypothesis: We hypothesize that early cessation of hydrocortisone in patients with septic shock would reduce composite adverse events including hyperglycemia, hypernatremia, muscle weakness, and the new onset of infection.
Ethics and dissemination: The trial receives ethical approval from Siriraj Hospital, Mahidol University (COA No.SI012/2023).
Trial registration: ClinicalTrials.govNCT05818826. Registered on April 19, 2023.
Downloads
References
Stephen MP. Steroids in the acutely ill: Evolving recommendations and practice. Cleveland Clinic Journal of Medicine. 2022;89:505.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Critical Care Medicine. 2021;49.
Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, et al. Hydrocortisone Therapy for Patients with Septic Shock. New England Journal of Medicine. 2008;358:111-24.
Keh D, Trips E, Marx G, Wirtz SP, Abduljawwad E, Bercker S, et al. Effect of Hydrocortisone on Development of Shock Among Patients With Severe Sepsis: The HYPRESS Randomized Clinical Trial. Jama. 2016;316:1775-85.
Rygård SL, Butler E, Granholm A, Møller MH, Cohen J, Finfer S, et al. Low-dose corticosteroids for adult patients with septic shock: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Medicine. 2018;44:1003-16.
Rochwerg B, Oczkowski SJ, Siemieniuk RAC, Agoritsas T, Belley-Cote E, D'Aragon F, et al. Corticosteroids in Sepsis: An Updated Systematic Review and Meta-Analysis. Crit Care Med. 2018;46:1411-20.
Venkatesh B, Finfer S, Cohen J, Rajbhandari D, Arabi Y, Bellomo R, et al. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. New England Journal of Medicine. 2018;378:797-808.
Solbakken G, Løseth S, Froholdt A, Eikeland TD, Nærland T, Frich JC, et al. Pain in adult myotonic dystrophy type 1: relation to function and gender. BMC Neurol. 2021;21:101.
Fang F, Zhang Y, Tang J, Lunsford LD, Li T, Tang R, et al. Association of Corticosteroid Treatment With Outcomes in Adult Patients With Sepsis: A Systematic Review and Meta-analysis. JAMA Intern Med. 2019;179:213-23.
Sobolewski KA, Brophy A, Opsha Y, Zaid A, Mistry N. Abrupt versus gradual cessation of steroids in patients with septic shock. Journal of Critical Care. 2018;48:198-202.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 The Thai Society of Critical Care Medicine

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.




