Early intravenous hydrocortisone in sepsis: A randomized control trial (Protocol)
Early intravenous hydrocortisone in sepsis
DOI:
https://doi.org/10.54205/ccc.v32.267616Keywords:
Corticosteroids, Sepsis, Hydrocortisone, ShockAbstract
Background: The evidence of the appropriate timing of hydrocortisone is still weak and controversial. Observational studies showed a trend towards greater benefits when hydrocortisone was given earlier in the course of septic shock resuscitation. This study evaluates the effects of early intravenous low-dose hydrocortisone administered at the beginning of the onset of sepsis-induced hypotension compared with standard care.
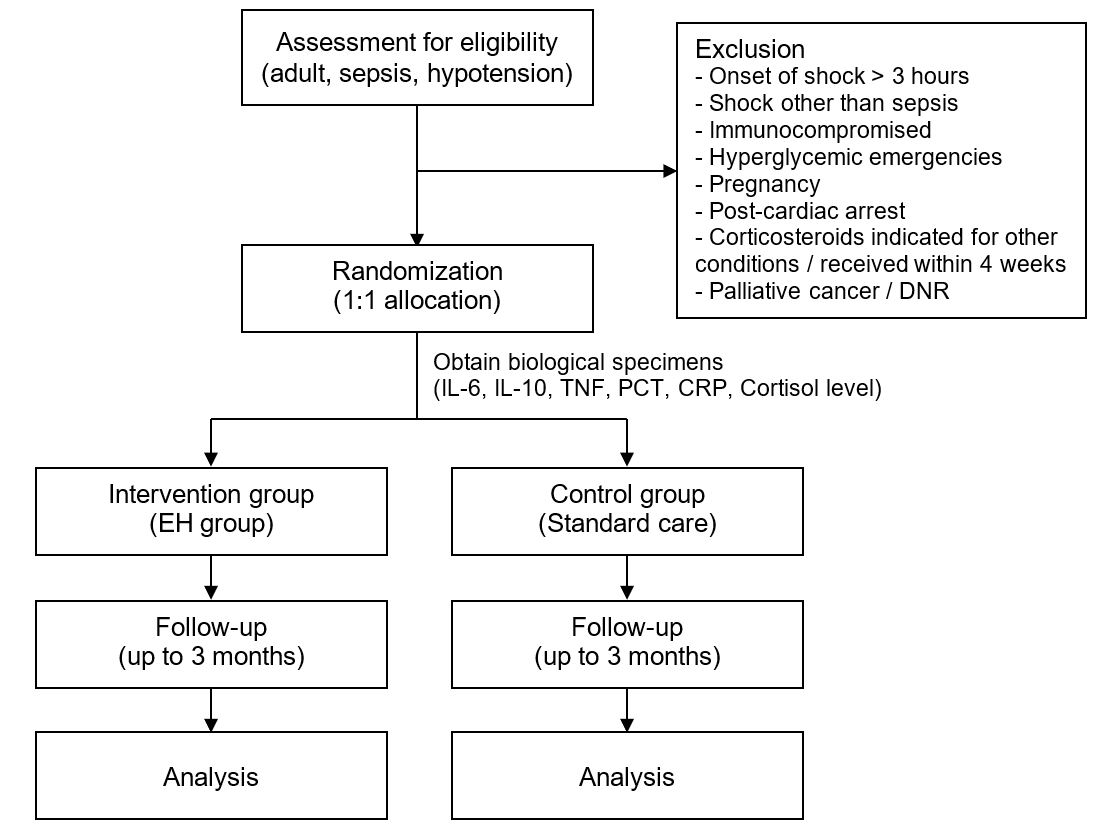
Methods: This study is a single-center, parallel-group, double-blinded, randomized control trial, conducted in a non-trauma emergency department. Adult patients with sepsis-induced hypotension will be included. Patients will be randomly assigned in a 1:1 ratio to receive early intravenous low-dose hydrocortisone or standard care. Blood inflammatory biomarkers at baseline will be collected. The primary outcome is 28-day mortality. Resuscitation-related secondary outcomes and safety outcomes will also be observed. Outcomes will be compared between groups. Subgroup analyses considering inflammatory biomarker levels will also be performed to evaluate the effect of early intravenous hydrocortisone, especially in patients with hyperinflammation.
Hypothesis: We hypothesize that early intravenous low-dose hydrocortisone administration in patients with sepsis-induced hypotension would result in less mortality and improve resuscitation outcomes, especially in subgroup of patients with hyperinflammation.
Ethics and dissemination: The study protocol was approved by the Siriraj Institutional Review Board with the certificate of approval number Si 917/2023.
Trial registration: Clinicaltrial.gov NCT06217939
Downloads
References
World Health Organisation. Global report on the epidemiology and burden of sepsis: current evidence, identifying gaps and future directions. 2020.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801-10.
Pirracchio R, Annane D, Waschka AK, Lamontagne F, Arabi YM, Bollaert PE, et al. Patient-level meta-analysis of low-dose hydrocortisone in adults with septic shock. NEJM Evid. 2023;2:EVIDoa2300034.
Rochwerg B, Oczkowski SJ, Siemieniuk RAC, Agoritsas T, Belley-Cote E, D'Aragon F, et al. Corticosteroids in sepsis: An updated systematic review and meta-analysis. Crit Care Med. 2018;46:1411-20.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181-247.
Annane D. Corticosteroids for severe sepsis: An evidence-based guide for physicians. Ann Intensive Care. 2011;1:7.
Ammar MA, Ammar AA, Wieruszewski PM, Bissell BD, M TL, Albert L, et al. Timing of vasoactive agents and corticosteroid initiation in septic shock. Ann Intensive Care. 2022;12:47.
Aldewereld ZT, Zhang LA, Urbano A, Parker RS, Swigon D, Banerjee I, et al. Identification of clinical phenotypes in septic patients presenting with hypotension or elevated lactate. Front Med (Lausanne). 2022;9:794423.
Seymour CW, Kennedy JN, Wang S, Chang CH, Elliott CF, Xu Z, et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA. 2019;321:2003-17.
Gardlund B, Dmitrieva NO, Pieper CF, Finfer S, Marshall JC, Taylor Thompson B. Six subphenotypes in septic shock: Latent class analysis of the PROWESS shock study. J Crit Care. 2018;47:70-9.
Bruno JJ, Dee BM, Anderegg BA, Hernandez M, Pravinkumar SE. US practitioner opinions and prescribing practices regarding corticosteroid therapy for severe sepsis and septic shock. J Crit Care. 2012;27:351-61.
Lv QQ, Gu XH, Chen QH, Yu JQ, Zheng RQ. Early initiation of low-dose hydrocortisone treatment for septic shock in adults: A randomized clinical trial. Am J Emerg Med. 2017;35:1810-4.
Venkatesh B, Finfer S, Cohen J, Rajbhandari D, Arabi Y, Bellomo R, et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018;378:797-808.
Annane D, Renault A, Brun-Buisson C, Megarbane B, Quenot JP, Siami S, et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. 2018;378:809-18.
Park HY, Suh GY, Song JU, Yoo H, Jo IJ, Shin TG, et al. Early initiation of low-dose corticosteroid therapy in the management of septic shock: A retrospective observational study. Crit Care. 2012;16:R3.
Ragoonanan D, Allen B, Cannon C, Rottman-Pietrzak K, Bello A. Comparison of early versus late initiation of hydrocortisone in patients with septic shock in the ICU setting. Ann Pharmacother. 2022;56:264-70.
Chan AW, Tetzlaff JM, Gotzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: Guidance for protocols of clinical trials. BMJ. 2013;346:e7586.
Gilboy N, Tanabe T, Travers D RA, Gilboy N Travers D, Rosenau A TP. Emergency Severity Index (ESI): A triage tool for Emergency Department Care, Version 4. Implementation Handbook 2012 Edition. AHRQ Publ No 12-00014 2011.
Permpikul C, Tongyoo S, Viarasilpa T, Trainarongsakul T, Chakorn T, Udompanturak S. Early use of norepinephrine in septic shock resuscitation (CENSER). A randomized trial. Am J Respir Crit Care Med. 2019;199:1097-105.
Goradia S, Sardaneh AA, Narayan SW, Penm J, Patanwala AE. Vasopressor dose equivalence: A scoping review and suggested formula. J Crit Care. 2021;61:233-40.
Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111-24.
Bernard R. Fundamentals of Biostatistics, (5th edition). Boston, PWS Publ 2000.
Moreno RP, Metnitz PG, Almeida E, Jordan B, Bauer P, Campos RA, et al. SAPS 3--From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31:1345-55.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373-83.
Schulz KF, Grimes DA. Multiplicity in randomised trials II: subgroup and interim analyses. Lancet. 2005;365:1657-61.
Zhao Y, Ding C. Effects of hydrocortisone on regulating inflammation, hemodynamic stability, and preventing shock in severe sepsis patients. Med Sci Monit. 2018;24:3612-9.
Marik PE. Procalcitonin is an essential biomarker for hydrocortisone, ascorbic acid, and thiamine (HAT) therapy in patients with sepsis. Crit Care. 2019;23:151.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 The Thai Society of Critical Care Medicine

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.




