Fluid bolus in suspected Sepsis patients with Hyperlactatemia (FISH): Study protocol for an open-labeled, randomized controlled trial
Fluid bolus in sepsis: RCT
DOI:
https://doi.org/10.54205/ccc.v32.268593Keywords:
Fluid therapy, Hyperlactatemia, Hypotension, Randomized controlled trial, SepsisAbstract
Background: The adequate preload was the goal of hemodynamic optimization for sepsis resuscitation. The fluid strategy in the early phase of sepsis is unclear.
Objective: To investigate the efficacy of a fluid bolus to prevent new-onset hypotension in suspected sepsis patients with hyperlactatemia (Point-of-care serum lactate 2-4 mmol/L).
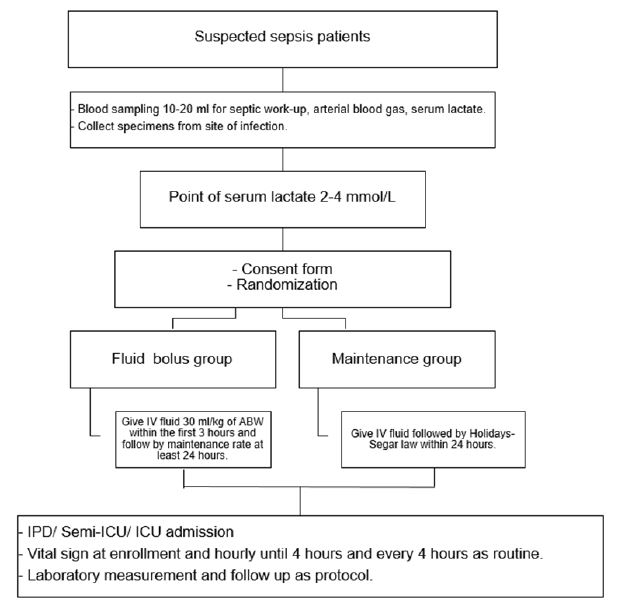
Methods: The Fluid Bolus in Suspected Sepsis Patients with Hyperlactatemia Trial (FISH) is a single-center, open-label randomized controlled trial. Participants will be patients suspected of having sepsis with hyperlactatemia (Point-of-care serum lactate 2-4 mmol/L) in the emergency department of Srinagarind Hospital, Thailand. Eligible patients will be randomized (1:1) to one of the study arms using block randomization. They will be placed in either the fluid bolus group (intervention, 30 mL/kg within 3 hours) or the standard care group (control). The primary outcome is new-onset hypotension within 24 hours after randomization. Secondary outcomes include lactate clearance, ∆SOFA at 72-hours, organ failure, and support ‘free days’ to day 28, 28-day mortality.
Hypothesis: We hypothesize that a fluid bolus will prevent new-onset hypotension in suspected sepsis patients with hyperlactatemia (point-of-care serum lactate 2-4 mmol/L).
Discussion: The optimal strategy for intravenous fluid therapy in a patient suspected of sepsis with hyperlactatemia is unknown. This is the first randomized trial examining fluid strategy in the early phase of sepsis with mild hyperlactatemia.
Ethics and dissemination: This study obtained approval from the Center for Ethics in Human Research at Khon Kaen University (Ethics Committee number: HE661012) and was registered at the Thai Clinical Trials Registry (TCTR20230502003).
Trial registration: TCTR20230502003
Downloads
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801-10.
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet. 2020;395:200-11.
World Health Organization. Report on the burden of endemic health care-associated infection worldwide, 2011. [cited 2024 March 16]. Available from: https://www.who.int/publications/i/item/report-on-the-burden-of-endemic-health-care-associated-infection-worldwide.
Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483-95.
Macdonald SPJ, Keijzers G, Taylor DM, Kinnear F, Arendts G, Fatovich DM, et al. Restricted fluid resuscitation in suspected sepsis associated hypotension (REFRESH): A pilot randomised controlled trial. Intensive Care Med. 2018;44:2070-8.
Chan AW, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: Guidance for protocols of clinical trials. BMJ. 2013;346:e7586.
Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372:1629-38.
Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683-93.
Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957;19:823-32.
Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301-11.
Oczkowski S, Alshamsi F, Belley-Cote E, Centofanti JE, Hylander Møller M, Nunnaly ME, et al. Surviving sepsis campaign guidelines 2021: Highlights for the practicing clinician. Pol Arch Intern Med. 2022;132.
Farrington CP, Manning G. Test statistics and sample size formulae for comparative binomial trials with null hypothesis of non-zero risk difference or non-unity relative risk. Stat Med. 1990;9:1447-54.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-81.
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208.
Meyhoff TS, Hjortrup PB, Wetterslev J, Sivapalan P, Laake JH, Cronhjort M, et al. Restriction of intravenous fluid in ICU patients with septic shock. N Engl J Med. 2022;386:2459-70.
Shapiro NI, Douglas IS, Brower RG, Brown SM, Exline MC, Ginde AA, et al. Early restrictive or liberal fluid management for sepsis-induced hypotension. N Engl J Med. 2023;388:499-510.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 The Thai Society of Critical Care Medicine

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.




