BASal-bolus Insulin therapy in Critically ill patients (BASIC): A randomized controlled trial protocol
Basal bolus insulin in critically ill patients
DOI:
https://doi.org/10.54205/ccc.v32.268831Keywords:
Basal bolus insulin, Sliding scale insulin, Critically ill patientAbstract
Background: Controlling blood glucose levels is crucial for optimizing outcomes in critically ill patients. While the sliding-scale insulin regimen is common, the efficacy of basal-bolus insulin therapy, using insulin glargine and insulin aspart, is less explored in critical care settings.
Objective: This study investigates the efficacy and safety of basal-bolus insulin therapy compared to sliding-scale insulin in managing hyperglycemia in critically ill patients in a medical intensive care unit (ICU).
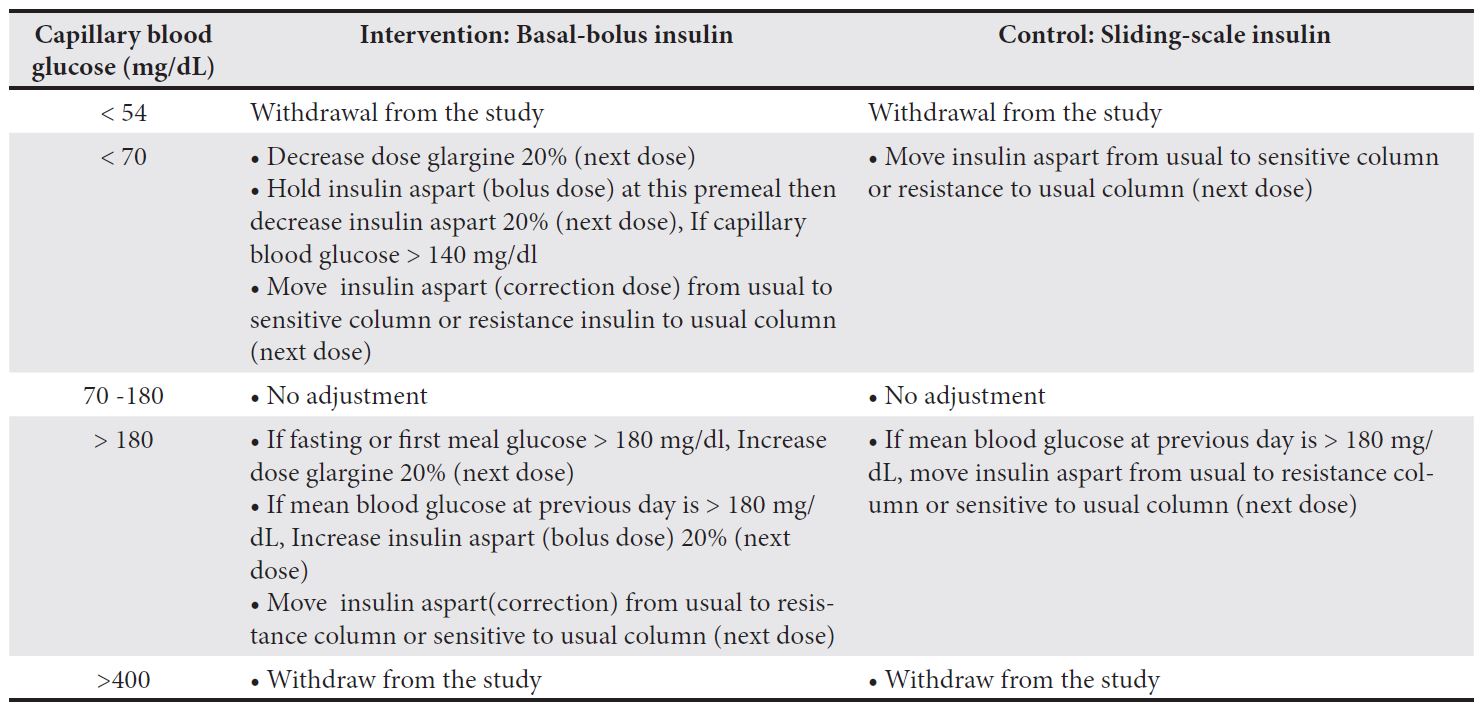
Methods: The BASal-Bolus Insulin Therapy in Critically Ill Patients (BASIC) trial is a single-center, open-label randomized controlled trial at Srinagarind Hospital, Thailand. The study will enroll adult critically ill patients admitted to the medical ICU with capillary blood glucose (CBG) levels between 180 and 400 mg/dL. Participants will be randomized (1:1) to receive either basal-bolus insulin therapy or sliding-scale insulin (control). The primary endpoint is the percentage of CBG within the target range of 140–180 mg/dL. The secondary outcomes include daily mean CBG levels, glucose variability index, 28-day mortality, length of stay in the ICU, incidence of nosocomial infections, ventilator-free days within 28 days, and occurrences of hypoglycemia.
Hypothesis: Basal-bolus insulin regimen has a higher efficacy in glycemic control compared to a sliding-scale regimen in critically ill medical patients.
Discussion: Evidence regarding the effectiveness of the basal-bolus insulin regimen in critically ill patients is limited, with most existing studies focusing on non-critically ill populations. This study addresses this gap by comparing the basal-bolus approach to the conventional sliding-scale insulin regimen. This trial aims to provide valuable insights into optimizing glycemic control in critically ill patients, potentially leading to improved clinical outcomes.
Ethics and dissemination: This study obtained approval from the Center for Ethics in Human Research at Khon Kaen University (Ethics Committee number: HE661013)
Trial registration: TCTR20230410009
Downloads
References
Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283-97.
Umpierrez GE, Smiley D, Zisman A, Prieto LM, Palacio A, Ceron M, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30:2181-6.
Tran MNT, Dang KT, Ly LD, Tran NQ. Basal-bolus insulin therapy for the treatment of non-critically ill patients with type 2 diabetes in Vietnam: effectiveness and factors associated with inpatient glycemic control. International Journal of Diabetes in Developing Countries. 2023;43:199-207.
van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359-67.
Falciglia M, Freyberg RW, Almenoff PL, D'Alessio DA, Render ML. Hyperglycemia-related mortality in critically ill patients varies with admission diagnosis. Crit Care Med. 2009;37:3001-9.
Chan AW, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586.
Jensen AK, Lange T. A novel high-power test for continuous outcomes truncated by death. arXiv preprint arXiv:1910.12267 2019.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-81.
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 The Thai Society of Critical Care Medicine

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.




