Factors Associated with False Positive Fecal Immunochemical Tests in Trang Colorectal Cancer Screening Program Population
Keywords:
Fecal immunochemical tests, Colorectal cancer, Screening, False positiveAbstract
Background and Objective: Early detection of colorectal cancer (CRC) has emerged as an important global issue. Since 2018, fecal immunochemical tests (FITs) have been offered as a primary screening test for CRC by the Thai National Health Service plan despite their varied accuracy according to various factors. The primary aim of this study was to identify demographic factors associated with false-positive (FP) FIT results in CRC screening in the Trang population. The secondary aim was to report the outcomes of the screening program in Trang Province.
Methods: The data of all 542 participants in Trang with positive FIT tests from the CRC screening program conducted at Trang Hospital between 1 October 2021 and 28 February 2023 were retrospectively reviewed. Of these, 347 with complete colonoscopy studies were analyzed. Patients’ characteristics, colonoscopy findings, and pathologic data were recorded. Univariable and multivariable logistic regression analysis was performed to determine factors associated with FP FIT results.
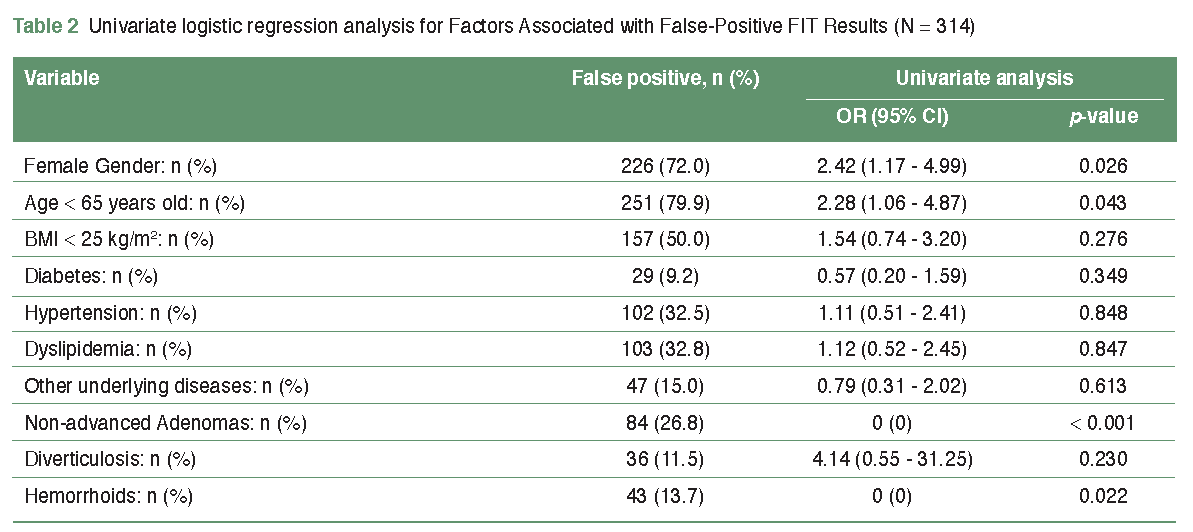
Results: Among 347 participants who showed positive FIT with complete colonoscopy for CRC screening, 33 participants (9.5%) had advanced colorectal neoplasia (ACRN), and 314 participants (90.5%) had FP FIT results (no ACRN). The participants aged under 65 had a higher rate of FP results than the older age group. Female gender and aged under 65 were two factors associated with false positivity. Multivariable logistic regression showed that age < 65 was the only independent risk factor associated with FP FIT results in multivariate analysis (OR = 2.81; 95% CI 1.24-6.35; p = 0.013).
Conclusion: FP FIT results in the Trang population were relatively high. The age < 65 is a significant factor associated with FP FIT results. This result can be used as a piece of evidence to optimize CRC screening strategies.
References
Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer, 2020. [cited 2023 May 7]. Available from: https://gco.iarc.fr/ today.
Lohsiriwat V, Chaisomboon N, Pattana-Arun J. Current colorectal cancer in Thailand. Ann Coloproctol. 2020;36:78-82. doi: 10.3393/ac.2020.01.07.
Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut 2015;64:1637-49. doi: 10.1136/gutjnl-2014-309086.
Monahan KJ, Davies MM, Abulafi M, et al. Faecal immunochemical testing (FIT) in patients with signs or symptoms of suspected colorectal cancer (CRC): a joint guideline from the Association of Coloproctology of Great Britain and Ireland (ACPGBI) and the British Society of Gastroenterology (BSG). Gut. 2022;71:1939-62. doi: 10.1136/gutjnl-2022-327985.
Amitay EL, Cuk K, Niedermaier T, et al, Brenner H. Factors associated with false-positive fecal immunochemical tests in a large German colorectal cancer screening study. Int J Cancer. 2019;144:2419-27. doi: 10.1002/ijc.31972.
de Klerk CM, Vendrig LM, Bossuyt PM, et al. Participant-related risk factors for false-positive and false-negative fecal immunochemical tests in colorectal cancer screening: systematic review and meta-analysis. Am J Gastroenterol. 2018;113:1778-87. doi: 10.1038/s41395-018-0212-7.
Symonds EL, Osborne JM, Cole SR, et al. Factors affecting fecal immunochemical test positive rates: demographic, pathological, behavioral, and environmental variables. J Med Screen. 2015;22:187-93. doi: 10.1177/0969141315584783.
Kim NH, Park JH, Park DI, et al. Are hemorrhoids associated with false-positive fecal immunochemical test result. Yonsei Med J. 2017;58:150-57. doi: 10.3349/ymj.2017.58.1.150.
Stegeman I, de Wijkerslooth TR, Stoop EM, et al. Risk factors for false positive and for false negative test results in screening with fecal occult blood testing. Int J Cancer. 2013; 133:2408-14. doi: 10.1002/ijc.28242.
Wong MC, Ching JY, Chan VC, et al. Factors associated with false-positive and false-negative fecal immunochemical test results for colorectal cancer screening. Gastrointest Endosc. 2015;81:596-607. doi: 10.1016/j.gie.2014.08.006.
Denter MJ, Deutekom M, Essink-Bot ML, et al. FIT false-positives in colorectal cancer screening experience psychological distress up to 6 weeks after colonoscopy. Support Care Cancer. 2013;21:2809-15. https://doi.org/10.1007/s00520-013-1867-7.
Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844-57. doi: 10.1053/j.gastro.2012.06.001.
Trang Provincial Public Health Office. Information to respond to the cancer service plan. Trang Health Data Center (HDC). Trang: Provincial Public Health Office. 2023. [cited 2023 May 7] Available from: https://trg.hdc.moph.go.th/hdc/reports/page.php?cat_id=59acae7a68f02c8e2c0cb88dfc6df3b3.
D'Souza N, Brzezicki A, Abulafi M. Faecal immunochemical testing in general practice. Br J Gen Pract. 2019;69:60-61. doi: 10.3399/bjgp19X700853.
Petersen MM, Kleif J, Jørgensen LN, et al. Optimizing Screening for Colorectal Cancer: An Algorithm Combining Fecal Immunochemical Test, Blood-Based Cancer-Associated Proteins and Demographics to Reduce Colonoscopy Burden. Clin Colorectal Cancer. 2023;22:199-210. doi: 10.1016/j.clcc.2023.02.001.
Rerknimit R, Kullavanijaya P, Treeprasertsuk S, et al. Feasibility of the Asia-Pacific colorectal cancer risk score and fetal immunochemical test for prioritization in colorectal neoplasm screening in Thailand: a multicenter study. Nonthaburi: Health System Research Institute; 2017.
Ministry of Public Health, Department of Medical Services. Guidelines for operation and data recording in colorectal cancer screening. Bangkok: National Cancer Institute, 2023.
Lee JK, Liles EG, Bent S, et al. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160:171. doi: 10.7326/M13-1484.
Lee Tj, Hull MA, Rajasekhar PT, et al. Aspirin users attending for NHS bowel cancer screening have less colorectal neoplasia: chemoprevention or false-positive faecal occult blood testing. Digestion. 2012;85:278-81. doi: 10.1159/000334372.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 The Royal College of Surgeons of Thailand

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Articles must be contributed solely to The Thai Journal of Surgery and when published become the property of the Royal College of Surgeons of Thailand. The Royal College of Surgeons of Thailand reserves copyright on all published materials and such materials may not be reproduced in any form without the written permission.


