In vitro tests to identification of culprit drugs for severe cutaneous drug reactions
Keywords:
Drug hypersensitivity, severe cutaneous adverse drug reactions, basophil activation test, lymphocyte transformation testAbstract
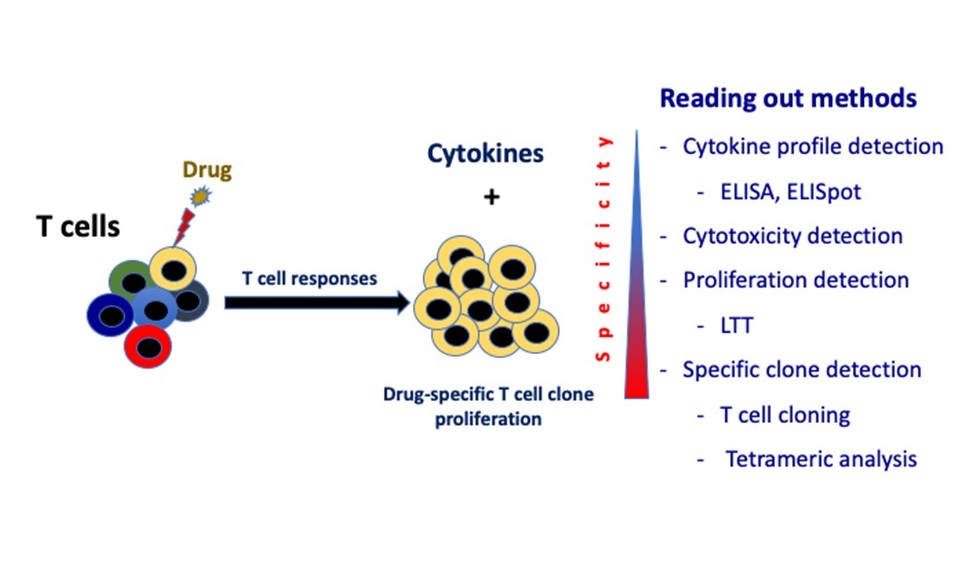
Drug hypersensitivity reactions (DHRs) are considered as an important public health problem because they can lead to life-threatening conditions. The DHRs occur in certain people and are often not predictable. The most commons of severe DHRs are anaphylaxis and severe cutaneous adverse drug reactions (SCARs) containing acute generalized exanthematous pustulosis, drug reaction with eosinophilia and systemic symptoms syndrome, and Stevens-Johnson syndrome/toxic epidermal necrolysis. Although clinical evaluation with causality assessment methods is a non-invasive method to define suspicious drug, the majority of assessment grading falls into probable or possible. Without dedicate investigation, it is difficult to identify culprit drug. As severe DHRs are life-threatening conditions, drug provocation test has not been recommended and other in vivo skin tests have to be performed cautiously. It has been recommended that in vitro tests (if available) could be performed prior to any in vivo tests. Therefore, in vitro diagnostic tests could be an alternative for SCARs diagnosis with culprit drug identification. As the most common of severe DHRs are immediate and delayed type hypersensitivity reactions, there are many tests approached to identify causative agents for both reactions such as ELISA, ELISpot, basophil activation test (BAT) and lymphocyte transformation test (LTT). Nevertheless, BAT and LTT are functional in vitro tests serve as more reliable among in vitro tests for immediate and delayed type hypersensitivity reactions. Both BAT and LTT has been performed and broadly available in many countries, including Thailand. They have been promising tests that contribute to management of SCARs in clinical practice.
References
Coombs P, Gell P. Classification of allergic reactions responsible for clinical hypersensitivity and disease. In: Gell R, editor. Clinical Aspects of Immunology. Oxford: Oxford University Press; 1968. p.575-96.
Pichler WJ, Adam J, Daubner B, Gentinetta T, Keller M, Yerly D. Drug hypersensitivity reactions: pathomechanism and clinical symptoms. Med Clin North Am 2010;94:645-64, xv.
Demoly P, Adkinson NF, Brockow K, et al. International Consensus on drug allergy. Allergy 2014;69:420-37.
Kuruvilla M, Khan DA. Anaphylaxis to drugs. Immunol Allergy Clin North Am 2015;35:303-19.
Doña I, Barrionuevo E, Blanca-Lopez N, et al. Trends in hypersensitivity drug reactions: more drugs, more response patterns, more heterogeneity. J Investig Allergol Clin Immunol 2014;24:143-53.
Torres MJ, Mayorga C, Blanca M. Nonimmediate allergic reactions induced by drugs: pathogenesis and diagnostic tests. J Investig Allergol Clin Immunol 2009;19:80-90.
Pichler W. Drug hypersensitivity: classification and relationship to T cell activation. In: Pichler W, editor. Drug hypersensitivity. Basel (Switzerland): Karger 2007:p168-89.
Hari Y, Urwyler A, Hurni M, et al. Distinct serum cytokine levels in drug- and measles-induced exanthema. Int Arch Allergy Immunol 1999;120:225-9.
Perkins JR, Ayuso P, Cornejo-García JA, Ranea JA. The study of severe cutaneous drug hypersensitivity reactions from a systems biology perspective. Curr Opin Allergy Immunol 2014;14:301-6.
Nassif A, Bensussan A, Dorothée G, et al. Drug specific cytotoxic T-cells in the skin lesions of a patient with toxic epidermal necrolysis. J Invest Dermatol 2002;118:728-33.
Schnyder B, Frutig K, Mauri-Hellweg D, Limat A, Yawalkar N, Pichler WJ. T-cell-mediated cytotoxicity against keratinocytes in sulfamethoxazol-induced skin reaction. Clin Exp Allergy 1998;28:1412-7.
Yawalkar N, Hari Y, Frutig K, et al. T cells isolated from positive epicutaneous test reactions to amoxicillin and ceftriaxone are drug specific and cytotoxic. J Invest Dermatol 2000;115:647-52.
Phillips EJ, Mallal SA. Pharmacogenetics of drug hypersensitivity. Pharmacogenomics 2010;11:973-87.
Britschgi M, Steiner UC, Schmid S, et al. T-cell involvement in drug-induced acute generalized exanthematous pustulosis. J Clin Invest 2001;107:1433-41.
Speeckaert MM, Speeckaert R, Lambert J, Brochez L. Acute generalized exanthematous pustulosis: an overview of the clinical, immunological and diagnostic concepts. Eur J Dermatol 2010;20:425-33.
Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy 2004;59:809-20.
Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239-45.
World Health Organization-Uppsala Monitoring Center. The use of the WHO-UMC system for standardized case causality assessment. Last accessed on February 1st, 2015: http://who-umc.org/Graphics/24734.pdf.
Romano A, Demoly P. Recent advances in the diagnosis of drug allergy. Curr Opin Allergy Clin Immunol 2007;7:299-303.
Elzagallaai AA, Rieder MJ. In vitro testing for diagnosis of idiosyncratic adverse drug reactions: Implications for Pathophysiology. Br J Clin Pharmacol 2015;80:889-900.
Fontaine C, Mayorga C, Bousquet PJ, et al. Relevance of the determination of serum-specific IgE antibodies in the diagnosis of immediate beta-lactam allergy. Allergy 2007;62:47-52.
Baldo BA, Pham NH. Adverse reactions to targeted and non-targeted chemotherapeutic drugs with emphasis on hypersensitivity responses and the invasive metastatic switch. Cancer Metastasis Rev 2013;32:723-61.
Steiner M, Harrer A, Himly M. Basophil Reactivity as Biomarker in Immediate Drug Hypersensitivity Reactions-Potential and Limitations. Front Pharmacol 2016;7:171.
Eberlein B, León Suárez I, Darsow U, Ruëff F, Behrendt H, Ring J. A new basophil activation test using CD63 and CCR3 in allergy to antibiotics. Clin Exp Allergy 2010;40:411-8.
Torres MJ, Romano A, Blanca-Lopez N, et al. Immunoglobulin E-mediated hypersensitivity to amoxicillin: in vivo and in vitro comparative studies between an injectable therapeutic compound and a new commercial compound. Clin Exp Allergy 2011;41:1595-601.
Aranda A, Mayorga C, Ariza A, et al. In vitro evaluation of IgE-mediated hypersensitivity reactions to quinolones. Allergy 2011;66:247-54.
Ben Said B, Berard F, Bienvenu J, Nicolas JF, Rozieres A. Usefulness of basophil activation tests for the diagnosis of IgE-mediated allergy to quinolones. Allergy 2010;65:535-6.
Hagau N, Gherman-Ionica N, Sfichi M, Petrisor C. Threshold for basophil activation test positivity in neuromuscular blocking agents hypersensitivity reactions. Allergy Asthma Clin Immunol 2013;9:42.
Leysen J, Bridts CH, De Clerck LS, et al. Allergy to rocuronium: from clinical suspicion to correct diagnosis. Allergy 2011;66:1014-9.
Pinnobphun P, Buranapraditkun S, Kampitak T, Hirankarn N, Klaewsongkram J. The diagnostic value of basophil activation test in patients with an immediate hypersensitivity reaction to radiocontrast media. Ann Allergy Asthma Immunol 2011;106:387-93.
Salas M, Gomez F, Fernandez TD, Dona I, Aranda A, Ariza A, et al. Diagnosis of immediate hypersensitivity reactions to radiocontrast media. Allergy 2013;68:1203-6.
Kim MS, Cho YJ. Flow Cytometry-Assisted Basophil Activation Test as a Safe Diagnostic Tool for Aspirin/NSAID Hypersenstivity. Allergy Asthma Immunol Res 2012;4:137-42.
Abuaf N, Rostane H, Barbara J, et al. Comparison of CD63 Upregulation Induced by NSAIDs on Basophils and Monocytes in Patients with NSAID Hypersensitivity. J Allergy (Cairo) 2012;2012:580873.
Rouzaire P, Nosbaum A, Denis L, et al. Negativity of the basophil activation test in quinolone hypersensitivity: a breakthrough for provocation test decision-making. Int Arch Allergy Immunol 2012;157:299-302.
Mayorga C, Celik G, Rouzaire P, et al. In vitro tests for drug hypersensitivity reactions: an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy 2016;71:1103-34.
Mayorga C, Ebo DG, Lang DM, et al. Controversies in drug allergy: In vitro testing. J Allergy Clin Immunol 2019;143:56-65.
Neukomm CB, Yawalkar N, Helbling A, Pichler WJ. T-cell reactions to drugs in distinct clinical manifestations of drug allergy. J Investig Allergol Clin Immunol 2001;11:275-84.
Hausmann O, Schnyder B, Pichler WJ. Drug hypersensitivity reactions involving skin. Handb Exp Pharmacol 2010;196:29-55.
Nyfeler B, Pichler WJ. The lymphocyte transformation test for the diagnosis of drug allergy: sensitivity and specificity. Clin Exp Allergy 1997;27:175-81.
Srinoulprasert Y, Pichler WJ. Enhancement of drug-specific lymphocyte proliferation using CD25(hi)-depleted CD3(+) effector cells. Int Arch Allergy Immunol 2014;163:198-205.
Cabañas R, Calderón O, Ramírez E, et al. Sensitivity and specificity of the lymphocyte transformation test in drug reaction with eosinophilia and systemic symptoms causality assessment. Clin Exp Allergy 2018;48:325-33.
Hari Y, Frutig-Schnyder K, Hurni M, et al. T cell involvement in cutaneous drug eruptions. Clin Exp Allergy 2001;31:1398-408.
Rozieres A, Vocanson M, Saïd BB, Nosbaum A, Nicolas JF. Role of T cells in nonimmediate allergic drug reactions. Curr Opinion Allergy Clin Immunol 2009;9:305-10.
Schmid DA, Depta JP, Pichler WJ. T cell-mediated hypersensitivity to quinolones: mechanisms and cross-reactivity. Clin Exp Allergy 2006;36:59-69.
Kano Y, Hirahara K, Mitsuyama Y, Takahashi R, Shiohara T. Utility of the lymphocyte transformation test in the diagnosis of drug sensitivity: dependence on its timing and the type of drug eruption. Allergy 2007;62:1439-44.
Porebski G. In Vitro Assays in Severe Cutaneous Adverse Drug Reactions: Are They Still Research Tools or Diagnostic Tests Already? Int J Mol Sci 2017;18:1737.
Kumkamthornkul P, Udnaen S, Tansit T, Tuchinda P, Srinoulprasert Y. Evaluation of a lymphocyte transformation test and cytokine detection assay to identify phenytoin and carbamazepine provoked DRESS or SJS/TEN in epilepsy patients. Int Immunopharmacol 2018;63:204-10.
Karami Z, Mesdaghi M, Karimzadeh P, et al. Evaluation of Lymphocyte Transformation Test Results in Patients with Delayed Hypersensitivity Reactions following the Use of Anticonvulsant Drugs. Int Arch Allergy Immunol 2016;170:158-62.
Kim EY, Seol JE, Choi JH, Kim NY, Shin JG. Allopurinol-induced severe cutaneous adverse reactions: A report of three cases with the HLA-B(*)58:01 allele who underwent lymphocyte activation test. Transl Clin Pharmacol 2017;25:63-66.
Bellón T, Rodríguez-Martín S, Cabañas R, et al. Assessment of drug causality in Stevens-Johnson syndrome/toxic epidermal necrolysis: Concordance between lymphocyte transformation test and ALDEN. Allergy 2019;75:956-9.
Hoffmann HJ, Santos AF, Mayorga C, et al. The clinical utility of basophil activation testing in diagnosis and monitoring of allergic disease. Allergy 2015;70:1393-405.
Sturm EM, Kranzelbinder B, Heinemann A, Groselj-Strele A, Aberer W, Sturm GJ. CD203c-based basophil activation test in allergy diagnosis: characteristics and differences to CD63 upregulation. Cytometry B Clin Cytom 2010;78:308-18.
Sturm GJ, Kranzelbinder B, Sturm EM, Heinemann A, Groselj-Strele A, Aberer W. The basophil activation test in the diagnosis of allergy: technical issues and critical factors. Allergy 2009;64:1319-26.
Suthumchai N, Srinoulprasert Y, Thantiworasit P, et al. The measurement of drug-induced interferon gamma-releasing cells and lymphocyte proliferation in severe cutaneous adverse reactions. J Eur Acad Dermatol Venereol 2018;32:992-98
Downloads
Published
How to Cite
Issue
Section
License
เนื้อหาและข้อมูลในบทความที่ลงตีพิมพ์ในวารสารโรคผิวหนัง ถือเป็นข้อคิดเห็นและความรับผิดชอบของผู้เขียนบทความโดยตรงซึ่งกองบรรณาธิการวารสาร ไม่จำเป็นต้องเห็นด้วย หรือร่วมรับผิดชอบใดๆ
บทความ ข้อมูล เนื้อหา รูปภาพ ฯลฯ ที่ได้รับการตีพิมพ์ในวารสารโรคผิวหนัง ถือเป็นลิขสิทธิ์ของวารสารฯ หากบุคคลหรือหน่วยงานใดต้องการนำทั้งหมดหรือส่วนหนึ่งส่วนใดไปเผยแพร่ต่อหรือเพื่อกระทำการใดๆ จะต้องได้รับอนุญาตเป็นลายลักอักษรจากบรรณาธิการวารสารโรคผิวหนังก่อนเท่านั้น


