Cutaneous Side Effects of Epidermal Growth Factor Receptor Inhibitors (PRIDE syndrome) in Patients with Non-small Cell Lung Cancer: A Cross-sectional Study from Hatyai Hospital, Southern Thailand
Keywords:
Afatinib, cutaneous adverse events, epidermal growth factor receptor inhibitors, erlotinib, gefitinib, non-small cell lung cancer, PRIDE syndromeAbstract
Background: PRIDE syndrome (Papulopustules and/or paronychia, Regulatory abnormalities of hair growth, Itching, and Dryness due to Epidermal growth factor receptor inhibitors) is cutaneous adverse events of epidermal growth factor receptor (EGFR) inhibitors. Numerous studies show a correlation between the presence and severity of PRIDE syndrome and tumor response. However, previous studies about cutaneous adverse events are still limited especially in Asian and Thailand.
Objectives: This study aims to investigate the cutaneous adverse events of EGFR inhibitors and to explore correlations between cutaneous adverse events with patient’s factors and tumor response.
Methods: A single center, retrospective cross-sectional study, conducted in Hatyai hospital from September 2014 to June 2020. All medical records from 74 patients with stage IV non-small cell lung cancer who received EGFR inhibitors were retrieved and analyzed.
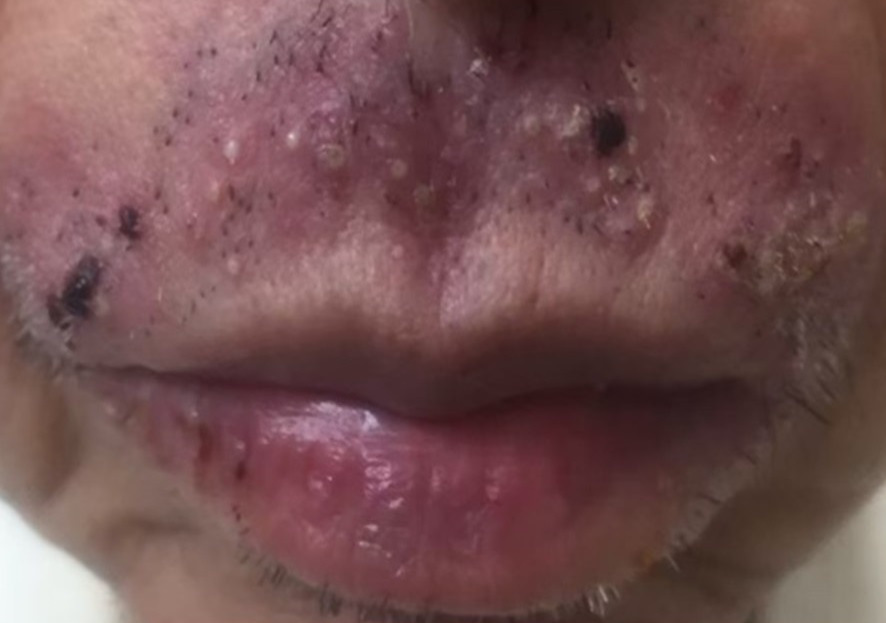
Results: In all 74 patients (53 females ,21 males), 41 (55.4%) patients received afatinib, 20 (27%) patients received gefitinib and 13 (17.6%) patients received erlotinib. Cutaneous adverse events occurred in 47 (63.5%) patients. The most common adverse events were xerosis (65.9%), paronychia (57.4%) and papulopustular rash (42.6%). PRIDE syndrome had statistically significant association with tumor response (P=0.001). However, there was no statistically significant association between patient’s factors and cutaneous adverse events.
Conclusions: PRIDE syndrome is common in Thais. The most common adverse events were xerosis. We also found statistically significant association between PRIDE syndrome and tumor response, supporting PRIDE syndrome can be used as a marker of tumor’s treatment outcome.
References
Lacouture ME, Lai SE. The PRIDE (Papulopustules and/or paronychia, Regulatory abnormalities of hair growth, Itching, and Dryness due to Epidermal growth factor receptor inhibitors) syndrome. Br J Dermatol 2006;155:852-4.
Madke B, Gole P, Kumar P, Khopkar U. Dermatological side effects of epidermal growth factor receptor inhibitors: ‘PRIDE’ complex. Indian J Dermatol 2014;59:271.
Peuvrel L, Bachmeyer C, Reguiai Z, Bachet JB, André T, Bensadoun RJ, et al. Semiology of skin toxicity associated with epidermal growth factor receptor (EGFR) inhibitors. Support Care Cancer 2012;20:909-21.
Kiyohara Y, Yamazaki N, Kishi A. Erlotinib-related skin toxicities: treatment strategies in patients with metastatic non-small cell lung cancer. J Am Acad Dermatol 2013;69:463-72.
Chanprapaph K, Vachiramon V, Rattanakaemakorn P. Epidermal growth factor receptor inhibitors: a review of cutaneous adverse events and management. Dermatol Res Pract 2014;2014:734249.
Agero AL, Dusza SW, Benvenuto-Andrade C, Busam KJ, Myskowski P, Halpern AC. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol 2006;55:657-70.
Giovannini M, Gregorc V, Belli C, Roca E, Lazzari C, Vigano MG, et al. Clinical significance of skin toxicity due to EGFR-targeted therapies. J Oncol 2009;2009:849051.
Lynch TJ Jr, Kim ES, Eaby B, Garey J, West DP, Lacouture ME. Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist 2007;12:610-21.
Chanprapaph K, Pongcharoen P, Vachiramon V. Cutaneous adverse events of epidermal growth factor receptor inhibitors: A retrospective review of 99 cases. Indian J Dermatol Venereol Leprol 2015;81:547.
Chularojanamontri L, Tuchinda P, Likitwattananurak C, Pongparit K, Rujitharanawong C, Ithimakin S, et al. Cutaneous toxicities of epidermal growth factor receptor inhibitors: A prospective study in 60 Asian patients. Asian Pac J Allergy Immunol 2019;37:12-8.
P´erez-Soler R. Can rash associated with HER1/EGFR inhibition be used as a marker of treatment outcome?. Oncology (Williston Park) 2003;17:23-8.
Liu HB, Wu Y, Lv TF, Yao YW, Xiao YY, Yuan DM, et al. Skin rash could predict the response to EGFR tyrosine kinase inhibitor and the prognosis for patients with non-small cell lung cancer: a systematic review and meta-analysis. PLoS One 2013;8:e55128.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sagen D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47.
Nanney LB, Stoscheck CM, King LE Jr. Underwood RA, Holbrook KA, et al. Immunolocalization of epidermal growth factor receptors in normal developing human skin. J Invest Dermatol 1990;94:742–48.
Lacouture ME, Basti S, Patel J, Benson A 3rd. The SERIES clinic: An interdisciplinary approach to the management of toxicities of EGFR inhibitors. J Support Oncol 2006;4:236-38.
Biswas B, Ghadyalpatil N, Krishna MV, Deshmukh J. A review on adverse event profiles of epidermal growth factor receptor-tyrosine kinase inhibitors in nonsmall cell lung cancer patients. Indian J Cancer 2017;541:S55-64.
Downloads
Published
How to Cite
Issue
Section
License
เนื้อหาและข้อมูลในบทความที่ลงตีพิมพ์ในวารสารโรคผิวหนัง ถือเป็นข้อคิดเห็นและความรับผิดชอบของผู้เขียนบทความโดยตรงซึ่งกองบรรณาธิการวารสาร ไม่จำเป็นต้องเห็นด้วย หรือร่วมรับผิดชอบใดๆ
บทความ ข้อมูล เนื้อหา รูปภาพ ฯลฯ ที่ได้รับการตีพิมพ์ในวารสารโรคผิวหนัง ถือเป็นลิขสิทธิ์ของวารสารฯ หากบุคคลหรือหน่วยงานใดต้องการนำทั้งหมดหรือส่วนหนึ่งส่วนใดไปเผยแพร่ต่อหรือเพื่อกระทำการใดๆ จะต้องได้รับอนุญาตเป็นลายลักอักษรจากบรรณาธิการวารสารโรคผิวหนังก่อนเท่านั้น


