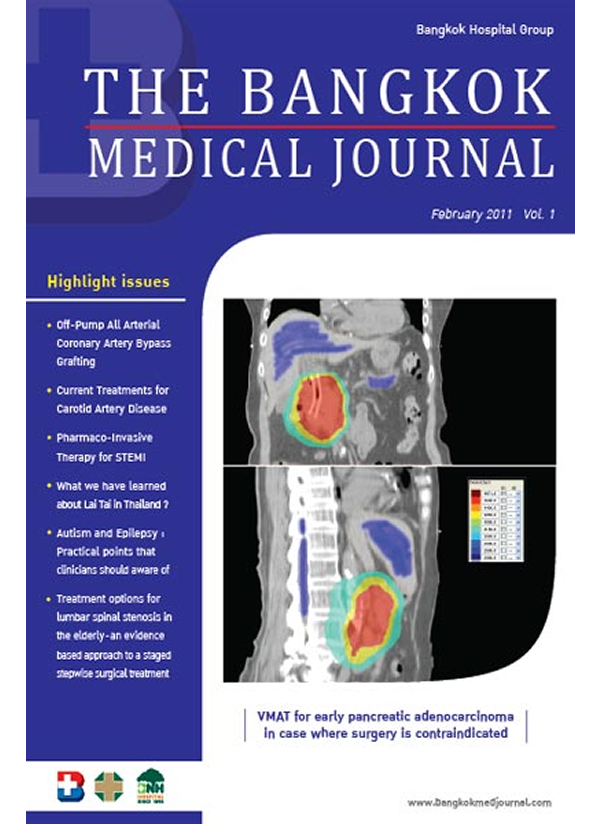
Drug Induced Cardio-vascular Events
Main Article Content
Abstract
Before drugs are launched on the market, studies should have already proven that these drugs are efficient in treatment of specific conditions, by trials in vivo, on experimental animals and then finally on humans.
Article Details
How to Cite
1.
Groodpanta S, Ieosriyoke U. Drug Induced Cardio-vascular Events. BKK Med J [internet]. 2018 Jan. 18 [cited 2026 Jan. 12];1(1):103. available from: https://he02.tci-thaijo.org/index.php/bkkmedj/article/view/217676
Issue
Section
Clinical Practice
This is an open access article distributed under the terms of the Creative Commons Attribution Licence, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
1. Scheen AJ. Cardiovascular Risk-Benefit Profile of Sibutramine. Am J Cardiovasc Drugs 2010;10:321-334
2. James WP, Caterson ID, Coutinho W, et al. Effect of Sibutramine on Cardiovascular Outcomes in Overweight and Obese Subjects. N Engl J Med 2010;363:905-917
3. Harrison-Woolrych M, Ashton J, Herbison P. Fatal and Non-Fatal Cardiovascular Events in a General Popula- tion Prescribed Sibutramine in New Zealand. Drug Saf 2010;33:605-613
4. Nissen SE, Wolski K. Effect of Rosiglitazone on the Risk of Myocardial Infarction and Death from Cardio- vascular Causes. N Engl J Med 2007;356:2457-2471
5. Nissen SE, Wolski K. Rosiglitazone Revisited: An updated Meta-analysis of Risk for Myocardial Infarction and Cardiovascular Mortality. Arch Intern Med 2010;170:1191-1201
6. Home PD, Pocock SJ, Beck-Nielsen H, et al; RECORD Study Team. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combi- nation therapy for type 2 diabetes (RECORD): a multicentre, randomized, open-label trial. Lancet 2009;373:2125-2135
7. US Food and Drug Administration. Guidance for industry diabetes mellitus - evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. http://www.fda.gov/downloads/Drugs/Guidance CompliacneRegulatoryInformation/Guidances/UCM 071627.pdf
2. James WP, Caterson ID, Coutinho W, et al. Effect of Sibutramine on Cardiovascular Outcomes in Overweight and Obese Subjects. N Engl J Med 2010;363:905-917
3. Harrison-Woolrych M, Ashton J, Herbison P. Fatal and Non-Fatal Cardiovascular Events in a General Popula- tion Prescribed Sibutramine in New Zealand. Drug Saf 2010;33:605-613
4. Nissen SE, Wolski K. Effect of Rosiglitazone on the Risk of Myocardial Infarction and Death from Cardio- vascular Causes. N Engl J Med 2007;356:2457-2471
5. Nissen SE, Wolski K. Rosiglitazone Revisited: An updated Meta-analysis of Risk for Myocardial Infarction and Cardiovascular Mortality. Arch Intern Med 2010;170:1191-1201
6. Home PD, Pocock SJ, Beck-Nielsen H, et al; RECORD Study Team. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combi- nation therapy for type 2 diabetes (RECORD): a multicentre, randomized, open-label trial. Lancet 2009;373:2125-2135
7. US Food and Drug Administration. Guidance for industry diabetes mellitus - evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. http://www.fda.gov/downloads/Drugs/Guidance CompliacneRegulatoryInformation/Guidances/UCM 071627.pdf