Motility degradation rate, plasma membrane integrity, and kinematics during cryopreservation of boar (Sus scrofa domesticus) spermatozoa in different freezing extenders and thawing temperatures https://doi.org/10.12982/VIS.2025.064
Main Article Content
Abstract
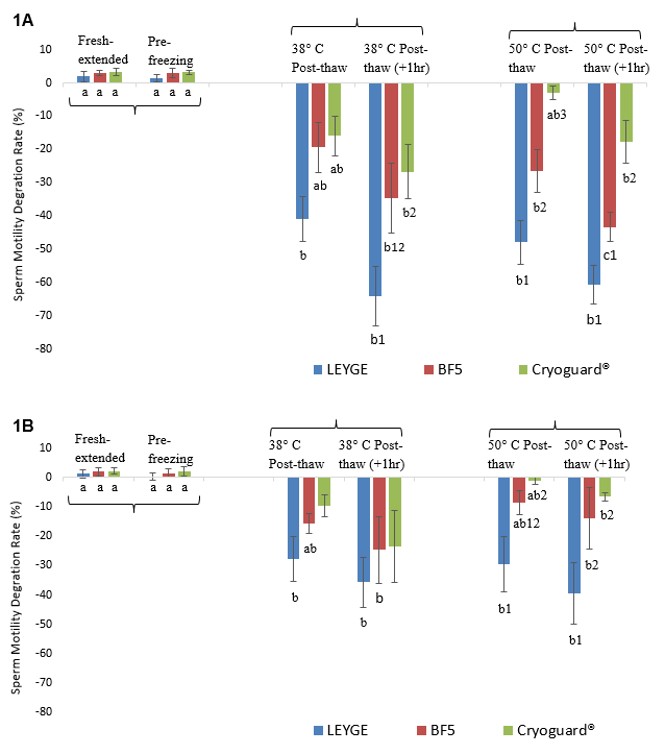
Despite the very limited use of frozen-thawed semen (FTS) in pig artificial insemination, FTS in some instances can be truly beneficial as it is not constrained by time (shelf-life) and space (regional quarantine) restrictions unlike fresh-extended semen (FES). It also allows long-term banking of highly valuable genetics particularly during epidemics. This study compares existing and currently available freezing extenders used in boar semen cryopreservation aimed to optimize protocols useful for in-country local swine industry with special focus on the motility degradation rate (MDR), and plasma membrane structural (percent live) and functional (HOST reactive) integrity. Treatment samples from ten freezing runs using five different sperm-rich fractions were frozen using three different cooling/freezing extenders (CE/FE): A) LEYGE, B) BF5, and C) Cryoguard (~500 x 106 spz/mL) in liquid nitrogen (LN2) vapor, thawed either at ~38°C or ~50°C for 20 sec, and examined using the Sperm Class Analyzer® CASA system. LEYGE had significantly the highest MDR from about 50% reduction post-thawing to 70% one hour thereafter. Cryoguard consistently had the lowest MDR although closely similar to BF5. There was a minimal effect on the plasma membrane functional integrity and was primarily limited to LEYGE and BF5. A fertility trial is recommended to attest the performance of FTS vs FES in terms of conception rates and the litter size following post-cervical AI before full-scale production and potential adoption by the breeder swine industry.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Publishing an article with open access in Veterinary Integrative Sciences leaves the copyright with the author. The article is published under the Creative Commons Attribution License 4.0 (CC-BY 4.0), which allows users to read, copy, distribute and make derivative works from the material, as long as the author of the original work is cited.
References
Bailey, J.L., Lessard, C., Jacques, J., Breque, C., Dobrinski, I., Zeng, W., Galantino-Homer, H.L., 2008. Cryopreservation of boar semen and its future importance to the industry. Theriogenology. 70, 1251-1259.
Baldaniya, R. V., Chaudhari, N. F., Modi, L. C., Patel, C. M., Puri, G., & Patel, J. M., 2020. Effect of coconut water in tris egg yolk citrate extender on cauda epididymal buck spermatozoa motility preserved at refrigeration temperature. Indian J. Anim. Hlth. 59, 55-61.
Casas, I., Althouse, G.C., 2013. The protective effect of a 17°c holding time on boar sperm plasma membrane fluidity after exposure to 5°c. Cryobiology. 66, 69-75.
Dinno, A., 2015. Nonparametric pairwise multiple comparisons in independent groups using Dunn's test. Stata J. 15, 292-300.
Holt, W.V., 2000. Basic aspects of frozen storage of semen. Anim. Reprod. Sci. 62, 3-22.
Jeyendran, R., Van der Ven, H., Zaneveld, L., 1992. The hypoosmotic swelling test: an update. Arch. Androl. 29, 105-116.
Knox, R., 2011a. The current value of frozen–thawed boar semen for commercial companies. Reprod. Domest. Anim. 46, 4-6.
Knox, R.V., 2011b. The current value of frozen–thawed boar semen for commercial companies. Reprod. Domest. Anim. 46, 4-6.
Knox, R., 2015. The fertility of frozen boar sperm when used for artificial insemination. Reprod. Domest. Anim. 50, 90-97.
Knox, R.V., 2016. Artificial insemination in pigs today. Theriogenology. 85, 83-93.
Lechniak, D., Kedzierski, A., Stanislawski, D., 2002. The use of HOS test to evaluate membrane functionality of boar sperm capacitated in vitro. Reprod. Domest. Anim. 37, 379-380.
Malo, C., Gil, L., Cano, R., Martínez, F., García, A., Jerez, R.A., 2012. Dimethylformamide is not better than glycerol for cryopreservation of boar semen. Andrologia. 44, 605-610.
Mortimer, S.T., 2000. Casa-practical aspects. J. Androl. 21, 515-524.
Peña, S.J.T., 2023. Boar sperm viability, head morphometry, and kinematics during seven-day storage in improvised portable semen shipper. Philipp J. Sci. 152, 535-549.
Peña, S.J.T., Janier, M.E.B., Ymas, B.T.P., 2024. Hypo-osmotic swelling test discriminates potentially more viable boar spermatozoa diluted using various extenders at different storage times. Vet. Integr. Sci. 22, 1127-1137.
Peña, S.J.T., Summers, P., Gummow, B., Paris, D.B.B.P., 2015. Oviduct binding ability of porcine spermatozoa develops in the epididymis and can be advanced by incubation with caudal fluid. Theriogenology. 83, 1502-1513.
Peña, S.J.T., Gummow, B., Parker, A.J., Paris, D.B., 2022. Boar spermatozoa maintains DNA integrity after cryopreservation using different concentrations of glycerol. Philipp. J. Vet Med. 59, 164-173.
Purdy, P.H., 2008. Ubiquitination and its influence in boar sperm physiology and cryopreservation. Theriogenology. 70, 818-826.
Pursel, V.G., Johnson, L.A., 1975. Freezing of boar spermatozoa: Fertilizing capacity with concentrated semen and a new thawing procedure. J. Anim. Sci. 40, 99-102.
Roca, J., Hernandez, M., Carvajal, G., Vazquez, J.M., Martinez, E.A., 2006. Factors influencing boar sperm cryosurvival. J. Anim. Sci. 84, 2692-2699.
Rodriguez-Martinez, H., 2006. State of the art in farm animal sperm evaluation. Reprod Fertil. Dev. 19, 91-101.
JASP Team, 2022. JASP (version 0.16.2). Available online: https://jasp-stats.org/.
Thurston, L.M., Watson, P.F., Mileham, A.J., Holt, W.V., 2001. Morphologically distinct sperm subpopulations defined by Fourier shape descriptors in fresh ejaculates correlate with variation in boar semen quality following cryopreservation. J. Androl. 22, 382-394.
van der Horst, G., Maree, L., du Plessis, S.S., 2018. Current perspectives of CASA applications in diverse mammalian spermatozoa. Reprod. Fertil. Dev. 30, 875-888.
Vazquez, J.M., Martinez, E.A., Martinez, P., Garcia-Artiga, C., Roca, J., 1997. Hypoosmotic swelling of boar spermatozoa compared to other methods for analysing the sperm membrane. Theriogenology. 47, 913-922.
Vyt, P., Maes, D., Quinten, C., Rijsselaere, T., Deley, W., Aarts, M., De Kruif, A., Soom, A., 2008. Detailed motility evaluation of boar semen and its predictive value for reproductive performance in sows. Vlaams Diergeneeskundig Tijdschrift. 77, 291-298.
Watson, P.F., 1995. Recent developments and concepts in the cryopreservation of spermatozoa and the assessment of their post-thawing function. Reprod. Fertil. Dev. 7, 871-891.
Yánez-Ortiz, I., Catalán, J., Rodríguez-Gil, J.E., Miró, J., Yeste, M., 2021. Advances in sperm cryopreservation in farm animals: Cattle, horse, pig and sheep. Anim. Reprod. Sci. 246, 106904.
Yeste, M., 2015. Recent advances in boar sperm cryopreservation: State of the art and current perspectives. Reprod. Domest. Anim. 50(Suppl 2), 71-79.
Yeste, M., Sancho, S., Briz, M., Pinart, E., Bussalleu, E., Bonet, S., 2010. A diet supplemented with l-carnitine improves the sperm quality of piétrain but not of duroc and large white boars when photoperiod and temperature increase. Theriogenology. 73, 577-586.