Evidence of histopathological appearances in representative fishes and invertebrates from Libong Island, Thailand https://doi.org/10.12982/VIS.2025.070
Main Article Content
Abstract
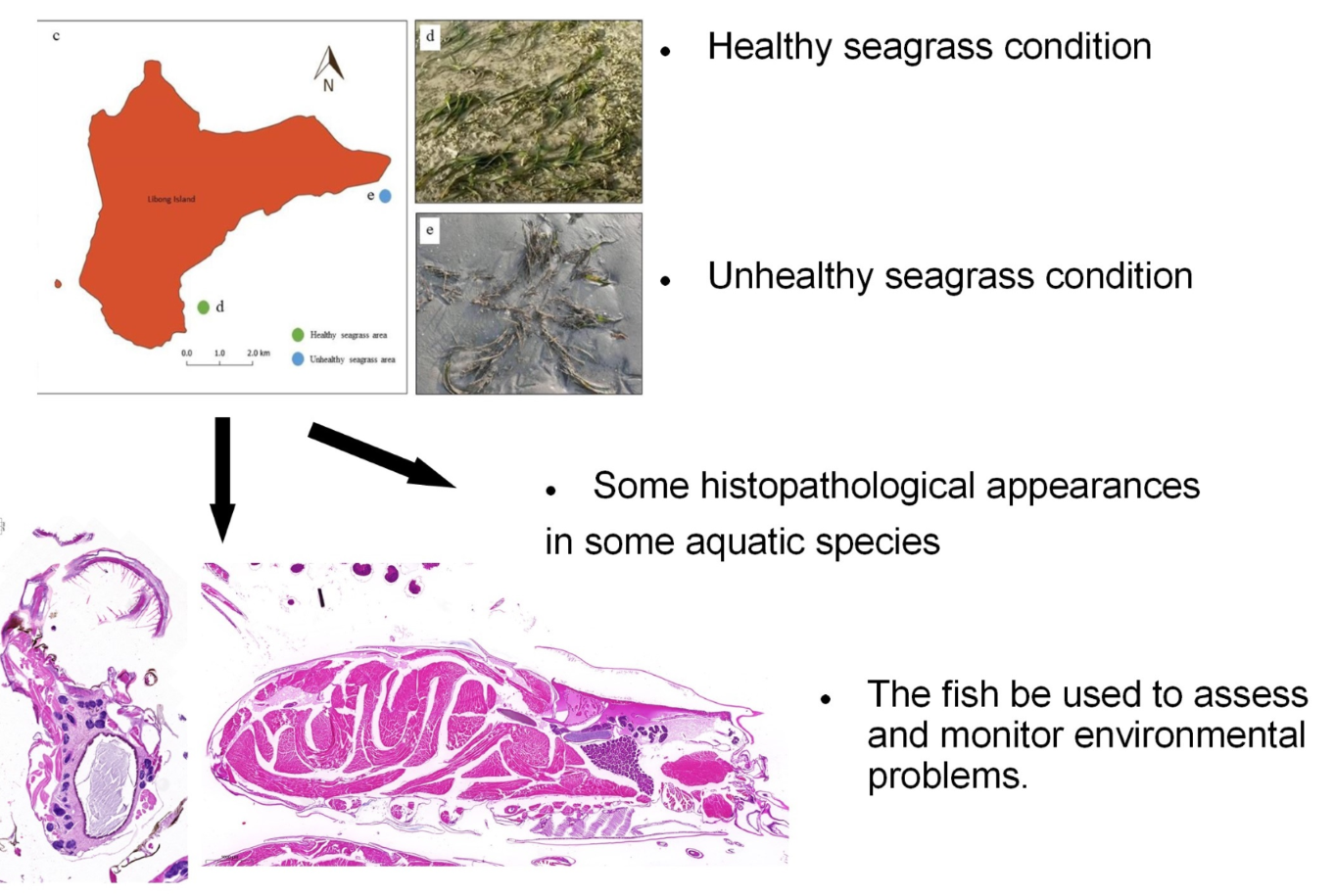
The seagrass beds at Libong Island, Thailand, are a complex ecological habitat supporting many marine organisms. Unfortunately, the seagrass area is being lost, possibly exerting adverse impacts on aquatic life, but comprehensive aquatic monitoring and assessment efforts are still lacking. In this study, sentinel species were selected from two species groups commonly found in this area, pelagic species (Ambassis nalua and A. vachelli) and benthic species (Amphibalanus amphitrite, and Alpheus sp.). Specimens were collected from healthy and unhealthy seagrass areas around the island from April to June 2021. The health of the specimens was assessed using the histopathological approach together with the histological alteration index (HAI). Some histological alterations were identified that HAl values indicated were significantly more prevalent in the unhealthy seagrass areas (P<0.05). Among the invertebrates, A. amphitrite exhibited melanomacrophage centers while Alpheus sp. exhibited lamellar disorganization in gill and degeneration of hepatopancreatic cells. The two fish species exhibited vacuolar degeneration in the liver that was more pronounced in specimens from the unhealthy seagrass area. However, the HAI values calculated for all samples ranged from 0.1 to 1, indicating normal organs. These results suggest the emergence of environmental alteration in the threatened seagrass habitats at Libong Island, where there is a need to monitor impacts on flora and fauna health in further studies. It is also noted that fishes can be sensitive sentinel species of aquatic ecosystem health.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Publishing an article with open access in Veterinary Integrative Sciences leaves the copyright with the author. The article is published under the Creative Commons Attribution License 4.0 (CC-BY 4.0), which allows users to read, copy, distribute and make derivative works from the material, as long as the author of the original work is cited.
References
Agius, C., Roberts, R.J., 2003. Melano-macrophage centres and their role in fish pathology. J. Fish Dis. 26, 499–509.
Ayas, Z., Ekmekci, G., Ozmen, M., Yerli, S.V., 2007. Histopathological changes in the livers and kidneys of fish in Sariyar Reservoir, Turkey. Environ. Toxicol. Pharmacol. 23, 242–249.
Barbierl, E., Rezende, K.F.O., Carneiro, J.S., Henriques, M.B., 2019. Metabolic and histological alterations after exposing Deuterodon iguape to different salinity. Bol. Inst. Pesca. 45, e.410.
Bhavan, P.S., Geraldine, P., 2000. Histopathology of the hepatopancreas and gills of the prawn Macrobrachium malcolmsonii exposed to endosulfan. Aqua. Toxicol. 50, 331–339.
Blazer, V.S., 2002. Histopathological assessment of gonadal tissue in wild fishes. Fish. Physiol. Biochem. 26, 85–101.
Braunbeck, T., 1998. Cytological alterations in fish hepatocytes following in vivo and in vitro sublethal exposure to xenobiotics - structural biomarkers of environmental contamination. In: Braunbeck, T., Hinton, D.E., Streit, B. (Eds.), Fish Ecotoxicology. Springer, Boston, pp. 61–140.
Carew, M.E., Pettigrove, V.J., Metzeling, L., Hoffmann, A.A., 2013. Environmental monitoring using next generation sequencing: rapid identification of macroinvertebrate bioindicator species. Front. Zool. 10, 45.
Ceccaldi, H.J., 1989. Anatomy and physiology of digestive tract of Crustaceans decapods reared in aquaculture. In: IFREMER (Ed.), Advances in Tropical Aquaculture, Workshop at Tahiti, French Polynesia, pp. 243–259.
Chiarelli, R., Roccheri, M.C., 2014. Marine invertebrates as bioindicators of heavy metal pollution. Open J. Met. 4, 93–106.
Dietrich, D.R., Krieger, H.O., 2009. Histological analysis of endocrine disruptive effects in small laboratory fish. John Wiley & Sons, New Jersey.
Dos Santos, I.V.F., de Souza, G.C., Santana, G.R., Duarte, J.L., Fernandes, C.P., Keita, H., Velázquez-Moyado, J.A., Navarrete, A., Ferreira, I.M., Carvalho, H.O., Carvalho, C.T., 2018. Histopathology in zebrafish (Danio rerio) to evaluate the toxicity of medicine: An anti-inflammatory phytomedicine with Janaguba Milk (Himatanthus drasticus Plumel). In: Srivastava, S. (Ed.), Histopathology - an update. Available online: https://www.intechopen.com/chapters/61145.
Dyková, I., Žák, J., Blažek, R., Reichard, M., Součková, K., Slabý, O., 2022. Histology of major organ systems of Nothobranchius fishes: short-lived model species. Available online: https://doi.org/10.25225/jvb.21074.
El-Gammal, M.A.M., Al-Madan, A., Fita, N., 2016. Shrimp, crabs and squids as bio-indicators for heavy metals in Arabian Gulf, Saudi Arabia. Int. J. Fish Aquat. Stud. 4, 200-207.
Ferguson, H.W., 1989. Systemic pathology of fish: a text and atlas of comparative tissue responses in diseases of teleosts. Iowa State University Press, Ames.
Fishelson, L., 1996. Ontogenesis and functional metamorphosis of the head-kidney in bottomspawner and mouthbrooder cichlid fishes (Cichlidae, Teleostei). J. Morphol. 229, 1-21.
Fowler, S.W., Teyssié, J.L., Cotret, O., Danis, B., Rouleau, C., Warnau, M., 2004. Applied radiotracer techniques for studying pollutant bioaccumulation in selected marine organisms (jellyfish, crabs and sea stars). Nukleonika. 49, 97−100.
Greenfield, B.K., Teh, S.J., Ross, J.R.M., Hunt, J., Zhang, G., Davis, J.A., Ichikawa, G., Crane, D., Hung, S.S.O., Deng, D., Teh F.C., Green, P.G., 2008. Contaminant concentrations and histopathological effects in Sacramento splittail (Pogonichthys macrolepidotus). Arch. Environ. Contam. Toxicol. 55, 270–281.
Hinton, D.E., Baumann, P.C., Gardner, G.R., Hawkins, W.E., Hendricks, J.D., Murchelano, R.A., Okihiro, M.S., 1992.
Histopathologic Biomarkers. In: Huggett, R.J., Klmerle, R.A., Mehrle, P.M., Bergman, H.L., Ward, C.H., Walton, B.T., LaPoint, T.W. (Eds.), Biomarkers: biochemical, physiological, and histological markers of anthropogenic stress, CRC Press, Boca Raton, pp. 155–209.
Hodkinson, I.D., Jackson, J.K., 2005. Terrestrial and aquatic invertebrates as bioindicators for environmental monitoring, with particular reference to mountain ecosystems. Environ. Man. 35, 649–666.
Laurén, D.J., Wails, D., 1990. Liver structural alterations accompanying chronic toxicity in fishes: Potential biomarkers of exposure. In: McCarthy, J.F., Shugart, L.R. (Eds.), Biomarkers of environmental contamination. CRC Press, Boca Raton, pp. 17–57.
Lazorchak, J.M., Hill, B.H., Brown, B.S., McCormick, F.H., Engle, V., Lattier, D.J., Bagley, M.J., Griffith, M.B., Maciorowski, A.F., Toth, G.P., 2003. USEPA biomonitoring and bioindicator concepts needed to evaluate the biological integrity of aquatic systems. In: Markert, B.A., Breure, A.M., Zechmeister, H.G. (Eds.), Bioindicators and biomonitors. Elsevier, Amsterdam, pp. 831-874.
Liebel, S., Tomotake, M.E.M., Ribeiro, C.A.O., 2013. Fish histopathology as biomarker to evaluate water quality. Ecotoxicol. Environ. Saf. 8, 9-15.
Lin, H.C., Wong, Y.H., Sung, C.H., Chan, B.K.K., 2021. Histology and transcriptomic analyses of barnacles with different base materials and habitats shed lights on the duplication and chemical diversification of barnacle cement proteins. BMC Genomics. 22, 783.
Lohaluksanadech, D., Somboon, K., Sawatprom, T., 2008. Analysis of water quality on coastal area of Rajamangala beach, Trang province. In Proceedings of 46th Kasetsart University Annual Conference, Bangkok, 29 January - 1 February 2008, pp. 517-525.
Maharajan, A., Narayanasamy, Y., Ganapiriya, V., Shanmugavel, K., 2015. Histological alterations of a combination of Chlorpyrifos and Cypermethrin (Nurocombi) insecticide in the freshwater crab, Paratelphusa jacquemontii (Rathbun). J. Basic. Applied. Zool. 72, 104-112.
Manrique, W.G., Claudiano da Silva, G., Petrillo, T.R., Pardi de Castro, M., Pereira Figueiredo, M.A., Belo de Andrade, M.A., Engracia de Moraes, J.R., Ruas de Moraes, F., 2014. Response of splenic melanomacrophage centers of Oreochromis niloticus (Linnaeus, 1758) to inflammatory stimuli by BCG and foreign bodies. J. Appl. Ichthyol. 30, 1001–1006.
Manrique, W.G., Pereira Figueiredo, M.A., Charlie-Silva, I., Antonio de Andrade Belo, M., Dib, C.C., 2019. Spleen melanomacrophage centers response of Nile tilapia during Aeromanas hydrophila and Mycobacterium marinum infections. Fish. Shellfish. Immunol. 95, 514–518.
National Research Council (NRC), 1991. Animals as sentinel of environmental health hazards. National Academy Press, Washington D.C.Organisation for Economic Co-operation and Development (OECD), 2009. OECD guidance document for the testing of chemicals. Available online: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd-gd125.pdf. (Accessed on December 23, 2018).
Paulo, D.V., Fontes, F.M., Flores-Lopes, F., 2012. Histopathological alterations observed in the liver of Poecilia vivipara (Cyprinodontiformes: Poeciliidae) as a tool for the environmental quality assessment of the Cachoeira River, BA. Braz. J. Biol. 72(1), 131 –140.
Penrith, M.L., Bastianello S.S., Penrith M.J., 1994. Hepatic lipidosis and fatty infiltration of organs in captive African stonefish, Synanceja verrucosa Bloch & Schneider. J. Fish. Dis. 17, 171–176.
Poleksic, V., Mitrovic-Tutundzic, V., 1994. Fish gills as a monitor of sublethal and chronic effects of pollution. In: Müller, R., Lloyd, R. (Eds.), Sublethal and chronic effects of pollutants on freshwater fish. Cambridge Univ Press, Cambridge, pp. 339-352.
Poolprasert, P., Senarat, S., Kettratad, J., Kaneko, G., Mongkolchaichana, E., Charoenphon, N., Thaochan, T., 2022. Comprehensive structure of the female marine water-strider Asclepios annandalei Distant, 1915 from Pranburi River Estuary, Thailand: New information for the Genus Asclepios. Trop. Life Sci. Res. 33, 47–60.
Pradit, S., Towatana, P., Nitiratsuwan, T., Jualaong, S., Jirajarus, M., Sornplang, K., Noppradit, P., Darakai, Y., Weerawong, C., 2020. Occurrence of microplastics on beach sediment at Libong, a pristine island in Andaman Sea, Thailand. ScienceAsia. 46, 336-343.
Presnell, J.K., Schreibman, M.P., 1997. Humason’s animal tissue techniques. Johns Hopkins University Press, Baltimore.
Reynoldson, T.B., Metcalfe-Smith, J.L., 1992. An overview of the assessment of aquatic ecosystem health using benthic invertebrates. Aquat. Ecosyst. Health Manag. 1, 295-308.
Robertson, J.C., Bradley, T.M., 1992. Liver ultrastructure of juvenile Atlantic salmon (Salmo salar). J. Morphol. 211, 41–54.
Sarojini, R., Reddy, P.S., Nagabhushanam, R., Fingerman, M., 1993. Napthalene-induced cytotoxicity on the hepatopancreatic cells of the red swamp crayfish, Procambarus clarkii. Bull. Environ. Contam. Toxicol. 51, 689–695.
Schrank, C.S., Cormier, S.M., Blazer, V.S., 1997. Contaminant exposure, biochemical, and histopathological biomarkers in white suckers from contaminated and reference sites in the Sheboygan River, Wisconsin. J. Great. Lakes. Res. 23, 119–130.
Senarat, S., Kettratad, J., Poolprasert, P., Jiraungkoorskul, W., Yenchum, W., 2015. Histopathological findings of liver and kidney tissues of the yellow mystus, Hemibagrus filamentus (Fang and Chaux, 1949), from the Tapee River, Thailand. Songklanakarin J. Sci. Technol. 37, 1–5.
Short, F.T., Wyllie-Echeverria, S., 1996. Natural and human induced disturbance in seagrass. Environ. Conser. 23, 17–27.
Soegianto, A., Charmantier-Daures, M., Trilles, J.P., Charmantier, G., 1999a. Impact of copper on the structure of gills and epipodites of the shrimp Penaeus japonicus. J. Crust. Biol. 19, 209–223.
Soegianto, A., Charmantier-Daures, M., Trilles, J.P., Charmantier, G. 1999b., Impact of cadmium on the structure of gills and epipodites of the shrimp Penaeus japonicus (Crustacea: Decapoda). Aquat. Living. Resour. 12, 57–70.
Spanò, L., Tylerm, C.R., van Aerl,e R., Devos, P., Mandiki, S.N.M., Silvestre,, S., Thome J.P., Kestemont, P., 2004. Effects of atrazine on sex steroid dynamics, plasma vitellogenin concentration and gonad development in adult goldfish (Carassius auratus). Aquatic. Toxicol. 66, 369–379.
Steinel, N.C., Bolnick, D.I., 2017. Melanomacrophage centers as a histological indicator of immune function in fish and other poikilotherms. Front. Immunol. 8, 827.
Suvarna, K.S., Layton, C., Bancroft, J.D., 2013. Bancroft’s theory and practice of histological techniques. Elsevier, Canada.
Tillitt, D.E., Papoulias, D.M., Whyte, J.J., Richter, C.A., 2010. Atrazine reduces reproduction in fathead minnow (Pimephales promelas). Aquatic Toxicol. 99, 149– 159.
Tjahjaningsih, W., Pursetyo, K.T., Sulmartiwi, L., 2017. Melanomacrophage centers in kidney, spleen and liver: a toxic response in carp fish (Cyprinus carpio) exposed to mercury chloride. AIP Conf. Proc. 1813, 020012.
Viarengo, A., 1993. Mussels as bioindicators in marine monitoring programs. In: Della Croce, N.F.R. (Ed.), Proceedings of the Symposium of the Mediterranean Seas. Santa Margherita Ligure, pp. 23–27.
Victor, B., 1993. Responses of hemocytes and gill tissues to sublethal cadmium chloride poisoning in the crab Paratelphusa hydrodromous (Herbst). Arch. Environ. Contam. Toxicol. 24, 432–439.
Wang, C., Schultzhaus, J.N., Taitt, C.R., Leary, D.H., Shriver-Lake, L.C., Snellings, D., Sturiale, S., North, S.H., Orihuela, B., Rittschof, D., Wahl, K.J., Spillmann, C.M., 2018. Characterization of longitudinal canal tissue in the acorn barnacle Amphibalanus amphitrite. PLoS ONE. 13, e0208352.
Wilson, J.M., Bunte, R.M., Carty, A.J., 2009. Evaluation of rapid cooling and tricaine methanesulfonate (MS 222) as methods of euthanasia in zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 48, 785–789.
Wirachwong, P., Sudtongkung, C., 2020. Sustainable spatial conservation project report for Dugong and seagrass: Grass mapping. Rajamangala University of Technology Srivijaya, Trang. Report.
Wolf, J.C., Wheeler, J.R., 2018. A critical review of histopathological findings associated with endocrine and non-endocrine hepatic toxicity in fish models. Aquat. Toxicol. 197, 60–78.