Influence of Electroporation Timing on CRISPR/Cas-Mediated Multiple Gene Editing in Buffalo Embryos https://doi.org/10.12982/VIS.2025.069
Main Article Content
Abstract
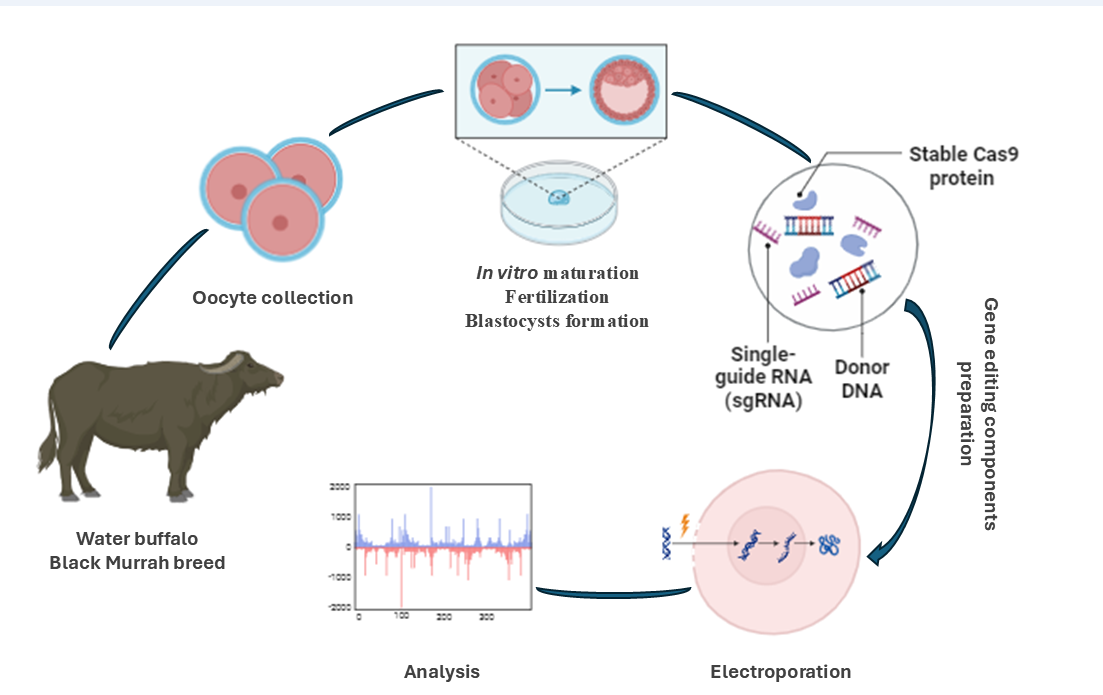
Gene editing in large animals like buffalo is challenged by mosaicism, where cells contain both wild-type and mutant alleles, complicating the creation of genetically modified F0 animals in a single step. Traditionally, electroporation is performed on zygotes post in vitro fertilization (IVF), but mature oocytes’ higher permeability suggests earlier intervention might reduce mosaicism and enhance editing efficiency. We hypothesized that the timing of electroporation before in vitro fertilization (IVF) can increase the rates of biallelic mutation for multiple gene knockout as the permeability of mature oocytes is greater than that of zygotes. Hence, we determined whether the timing of electroporation during in vitro maturation (IVM) culture enhances triple gene editing in the resulting blastocysts. Three gRNAs targeting KDR, GDF9, and POU5F1 were simultaneously introduced into the oocytes that had been incubated for 44, 46, and 48 h from the start of the IVM culture. Electroporation with three gRNAs at 44 h and 46 h during IVM culture decreased the blastocyst formation rates and did not improve the mutation rates and target number of biallelic mutations in the resulting blastocysts. The blastocyst formation rate, mutation rates, and target numbers in the resulting blastocysts from oocytes treated by electroporation at 48 h of IVM culture were similar to those of control zygotes electroporated at 12 h after the initiation of IVF. In conclusion, multiple gene editing efficiency in the resulting blastocysts was comparable between oocytes electroporated before and after fertilization, indicating that oocytes with completed maturation time may allow better functioning of materials accepting gene editing application.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Publishing an article with open access in Veterinary Integrative Sciences leaves the copyright with the author. The article is published under the Creative Commons Attribution License 4.0 (CC-BY 4.0), which allows users to read, copy, distribute and make derivative works from the material, as long as the author of the original work is cited.
References
Belli, M., Vigone, G., Merico, V., Redi, C.A., Garagna, S., Zuccotti, M., 2014. Time-lapse dynamics of the mouse oocyte chromatin organization during meiotic resumption. BioMed Res. Int. 2014(1), 207357.
Castro, F.C.D., Cruz, M.H.C., Leal, C.L.V., 2016. Role of growth differentiation factor 9 and bone morphogenetic protein 15 in ovarian function and their importance in mammalian female fertility-a review. Asian-Australas. J. Anim. Sci. 29(8), 1065-1074.
Chang, N., Sun, C., Gao, L., Zhu, D., Xu, X., Zhu, X., Xiong, J.W., Xi, J.J., 2013. Genome editing with RNA-guided Cas9 nuclease in Zebrafish embryos. Cell Res. 23(4), 465–472.
Civelek, M., Lusis, A.J., 2014. Systems genetics approaches to understand complex traits. Nat. Rev. Genet. 15(1), 34–48.
Escriba, M.J., Garcı́a-Ximénez, F., 2000. Influence of sequence duration and number of electrical pulses upon rabbit oocyte activation and parthenogenetic in vitro development. Anim. Reprod. Sci. 59(1-2), 99-107.
Foulkes, W.D., Real, F.X., 2013. Many mosaic mutations. Curr. Oncol. 20(2), 85–87.
Funahashi, H., 2003. Polyspermic penetration in porcine IVM-IVF systems. Reprod. Fertil. Dev. 15(3–4), 167–177.
Hashimoto, M., Yamashita, Y., Takemoto, T., 2016. Electroporation of Cas9 protein/sgRNA into early pronuclear zygotes generates non-mosaic mutants in the mouse. Dev. Biol. 418(1), 1–9.
Hirata, M., Tanihara, F., Wittayarat, M., Hirano, T., Nguyen, N.T., Le, Q.A., Namula, Z., Nii, M., Otoi, T., 2019. Genome mutation after introduction of the gene editing by electroporation of Cas9 protein (GEEP) system in matured oocytes and putative zygotes. In Vitro Cell. Dev. Biol. Anim. 55(4), 237–242.
Hsu, P.D., Lander, E.S., Zhang, F., 2014. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 157(6), 1262-1278.
Im, W., Moon, J., Kim, M., 2016. Applications of CRISPR/Cas9 for gene editing in hereditary movement disorders. J. Mov. Disord. 9(3), 136–143.
Kadoom, A.K., Abdel-Khalek, A.E., Shamiah, S.M., El-Sharawy, M.E., Abd ElRazek, I.M., 2014. In vitro maturation, fertilization and development of prepubertal and mature buffalo oocytes. Egypt. J. Anim. Prod. 51(2), 65-69.
Kikuchi, K., Nagai, T., Motlik, J., Shioya, Y., Izaike, Y., 1993. Effect of follicle cells on in vitro fertilization of pig follicular oocytes. Theriogenology. 39(3), 593–599.
Lin, H., Deng, Q., Li, L., Shi, L., 2019. Application and development of CRISPR/cas9 technology in pig research. Available online: https://www.intechopen.com/ chapters/67086 .
Liu, M., 2020. Research progress of CRISPR-Cas system application. Chronic Dis. Prev. Rev. 15, 1–4.
Liu, Y., Yu, M.C., Zhang, A.Q., Wang, Y.B., Jiang, K., Dong, J.H., 2015. Interleukin-10 gene promoter polymorphism and risk of liver cirrhosis. Genet. Mol. Res. 14(1), 1229–1234.
Marin, D.F.D., de Souza, E.B., de Brito, V.C., Nascimento, C.V., Ramos, A.S., Filho, S.T.R., da Costa, N.N., Cordeiro, M.D.S., Santos, S.D.S.D., Ohashi, O.M., 2019. In vitro embryo production in buffaloes: From the laboratory to the farm. Anim. Reprod. 16(2), 260–266.
Michelizzi, V.N., 2010. Whole Genome Single Nucleotide Polymorphism (SNP) transfer from cattle to water buffalo (Doctoral dissertation). Washington State University.
Nánássy, L., Lee, K., Jávor, A., Macháty, Z., 2008. Effects of activation methods and culture conditions on development of parthenogenetic porcine embryos. Anim. Reprod. Sci. 104(2–4), 264–274.
Ferrara, N., Gerber, H.P., 2001. The role of vascular endothelial growth factor in angiogenesis. Acta. Haematol. 106(4), 148-156.
Nguyen, T.V., Tanihara, F., Do, L.T.K., Sato, Y., Taniguchi, M., Takagi, M., Van Nguyen, T., Otoi, T., 2017. Chlorogenic acid supplementation during in vitro maturation improves maturation, fertilization and developmental competence of porcine oocytes. Reprod. Domest. Anim. 52(6), 969–975.
Prescott, M.J., 2020. Ethical and welfare implications of genetically altered non-human primates for biomedical research. J. Appl. Anim. Ethics Res. 2(2), 151–176.
Punetha, M., Kumar, D., Saini, S., Chaudhary, S., Bajwa, K.K., Sharma, S., Mangal, M., Yadav, P.S., Green, J.A., Whitworth, K., Datta, T.K., 2024. Optimising electroporation condition for CRISPR/Cas-mediated knockout in zona-intact buffalo zygotes. Animals. 14(1), 134.
Singh, B., Chauhan, M.S., Singla, S.K., Gautam, S.K., Verma, V., Manik, R.S., Singh, A. K., Sodhi, M., Mukesh, M., 2009. Reproductive biotechniques in buffaloes (Bubalus bubalis): status, prospects and challenges. Reprod. Fertil. Dev. 21(4), 499–510.
Singh, B., Mal, G., Kues, W.A., Yadav, P.S., 2020. The domesticated buffalo - An emerging model for experimental and therapeutic use of extraembryonic tissues. Theriogenology. 151, 95–102.
Suzuki, T., Asami, M., Perry, A.C.F., 2014. Asymmetric parental genome engineering by Cas9 during mouse meiotic exit. Sci. Rep. 4(1), 7621.
Van Blerkom, J., 2011. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 11(5), 797-813.
Wang, H., La Russa, M., Qi, L.S., 2016. CRISPR/Cas9 in genome editing and beyond. Annu. Rev. Biochem. 85(1), 227-264.
Wongsrikeao, P., Kaneshige, Y., Ooki, M., Taniguchi, M., Agung, B., Nii, M., Otoi, T., 2005. Effect of the removal of cumulus cells on the nuclear maturation, fertilization and development of porcine oocytes. Reprod. Domest. Anim. 40(2), 166–170.
Wu, X., Shen, W., Zhang, B., Meng, A., 2018. The genetic program of oocytes can be modified in vivo in the zebrafish ovary. J. Mol. Cell Biol. 10(6), 479–493.
Xu, Y., Li, Z., 2020. CRISPR-Cas systems: overview, innovations and applications in human disease research and gene therapy. Comput. Struct. Biotechnol. J. 18, 2401–2415.
Yadav, D.K., Kumar, S., Choi, E.H., Kim, M.H., 2021. Electric-field-induced electroporation and permeation of reactive oxygen species across a skin membrane. J. Biomol. Struct. Dyn. 39(4), 1343–1353.
Yang, D., Xu, J., Zhu, T., Fan, J., Lai, L., Zhang, J., Chen, Y.E., 2014. Effective gene targeting in rabbits using RNA-guided Cas9 nucleases. J. Mol. Cell Biol. 6(1), 97-99.
Yang, S., Li, S., Li, X.J., 2018. Shortening the half-life of Cas9 maintains its gene editing ability and reduces neuronal toxicity. Cell Rep. 25(10), 2653–2659.e3.
Yang, Z., Liu, D., 2021. Enhanced transmembrane transport of reactive oxygen species by electroporation effect of plasma. Plasma Process. Polym. 18(11), 2100054.
Yoshida, N., Brahmajosyula, M., Shoji, S., Amanai, M., Perry, A.C.F., 2007. Epigenetic discrimination by mouse metaphase II oocytes mediates asymmetric chromatin remodeling independently of meiotic exit. Dev. Biol. 301(2), 464–477.
Zhang, F., Wen, Y., Guo, X., 2014. CRISPR/Cas9 for genome editing: progress, implications and challenges. Hum. Mol. Genet. 23(R1), R40-R46.