In vitro differentiation of feline amniotic-derived mesenchymal stem cells into proximal tubular-like cells: A pilot study https://doi.org/10.12982/VIS.2026.025
Main Article Content
Abstract
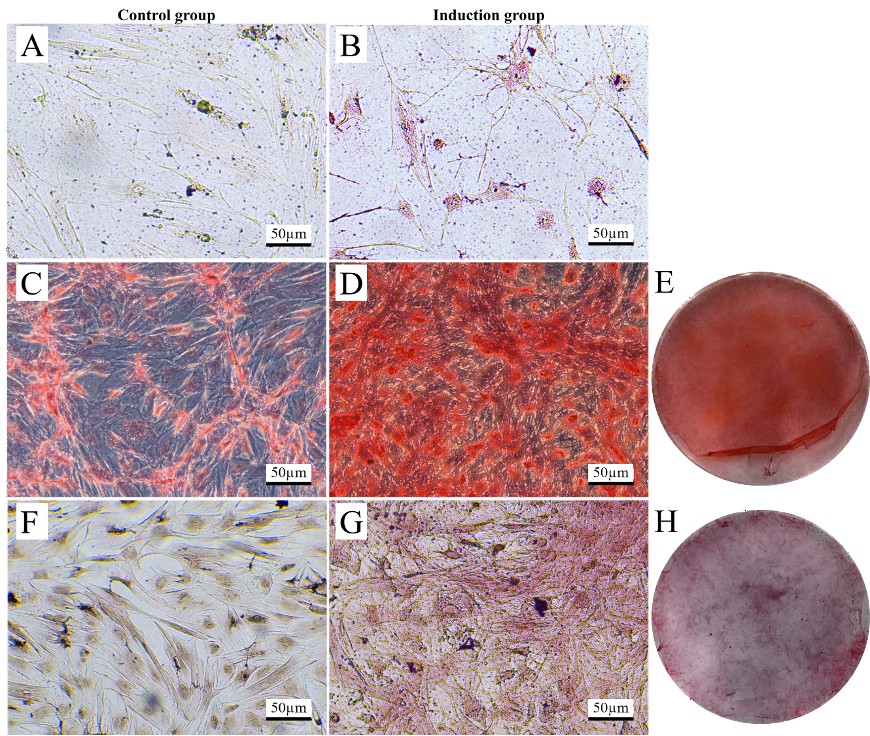
This study aims to isolate and characterize mesenchymal stem cells (MSCs) from the feline amniotic membrane and explore their potential for differentiation into proximal tubular-like cells (PTLCs) for regenerative medicine applications. The feline amniotic membrane-derived MSCs (AM-MSCs) from feline amniotic membranes were isolated successfully and characterized in terms of viability, morphology, and key MSC markers like CD73 and CD90. Exhibiting a shape reminiscent of fibroblasts, these cells also had a viability above 80% and could differentiate into adipogenic, chondrogenic, and osteogenic lineages after 21 days. Notably, the lack of hematopoietic indicators (CD34 and CD45) confirmed their mesenchymal lineage. The Differentiation into PTLCs was accomplished utilizing a protocol that incorporated Rho-Kinase (ROCK) inhibitor and specific growth factors, resulting in morphological alterations and gene expression reflective of PTLC characteristics. The upregulation of nephron-specific genes, such as Paired box gene 2 (PAX2), Gene Aquaporin 1 (AQP1), and Gamma-glutamyltransferase 1 (GGT), corroborated successful differentiation towards renal cell types, albeit with discrepancies among samples. These discoveries underscore the potential of feline AM-MSCs for the advancement of innovative therapeutic strategies for chronic kidney disease (CKD) in felines. Subsequent research should concentrate on refining differentiation protocols and undertaking in vivo assessments to ascertain their clinical efficacy in treating CKD and associated ailments in felines and potentially other species
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Publishing an article with open access in Veterinary Integrative Sciences leaves the copyright with the author. The article is published under the Creative Commons Attribution License 4.0 (CC-BY 4.0), which allows users to read, copy, distribute and make derivative works from the material, as long as the author of the original work is cited.
References
Abarca-Buis, R.F., Mandujano-Tinoco, E.A., Cabrera-Wrooman, A., Krotzsch, E., 2021. The complexity of TGFbeta/activin signaling in regeneration. J. Cell Commun. Signal. 15, 7-23.
Adan, A., Alizada, G., Kiraz, Y., Baran, Y., Nalbant, A., 2017. Flow cytometry: basic principles and applications. Crit. Rev. Biotechnol. 37, 163-176.
Ambrosio, C.E., Orlandin, J.R., Oliveira, V.C., Motta, L.C.B., Pinto, P.A.F., Pereira, V.M., Padoveze, L.R., Karam, R.G., Pinheiro, A.O., 2020. Potential application of aminiotic stem cells in veterinary medicine. Anim. Reprod. 16, 24-30.
Aronson, L.R., 2016. Update on the current status of kidney transplantation for chronic kidney disease in animals. Vet. Clin. North Am. Small Anim. Pract. 46, 1193-1218.
Bach, L.A., Hale, L.J., 2015. Insulin-like growth factors and kidney disease. Am. J. Kidney Dis. 65, 327-336.
Chan, Y.H., Ho, K.N., Lee, Y.C., Chou, M.J., Lew, W.Z., Huang, H.M., Lai, P.C., Feng, S.W., 2022. Melatonin enhances osteogenic differentiation of dental pulp mesenchymal stem cells by regulating MAPK pathways and promotes the efficiency of bone regeneration in calvarial bone defects. Stem Cell Res. Ther. 13, 73.
Chandrasekaran, V., Carta, G., da Costa Pereira, D., Gupta, R., Murphy, C., Feifel, E., Kern, G., Lechner, J., Cavallo, A.L., Gupta, S., Caiment, F., Kleinjans, J.C.S., Gstraunthaler, G., Jennings, P., Wilmes, A., 2021. Generation and characterization of iPSC-derived renal proximal tubule-like cells with extended stability. Sci. Rep. 11, 11575.
Choi, E.W., et, al., 2014. Feline mesenchymal stem cells derived from amniotic fluid and their potential for differentiation. J. Vet. Sci. 15, 515–522.
Cianci, R., Simeoni, M., Cianci, E., De Marco, O., Pisani, A., Ferri, C., Gigante, A., 2023. Stem cells in kidney ischemia: from inflammation and fibrosis to renal tissue regeneration. Int. J. Mol. Sci. 24, 4631.
de Ruiter, R.D., Wisse, L.E., Schoenmaker, T., Yaqub, M., Sanchez-Duffhues, G., Eekhoff, E.M.W., Micha, D., 2023. TGF-Beta induces activin A production in dermal fibroblasts derived from patients with Fibrodysplasia ossificans progressiva. Int. J. Mol. Sci. 24, 2299.
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., Deans, R., Keating, A., Prockop, D., Horwitz, E., 2006. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 8, 315-317.
Flyvbjerg, A., Khatir, D.S., Jensen, L.J., Dagnaes-Hansen, F., Gronbaek, H., Rasch, R., 2004. The involvement of growth hormone (GH), insulin-like growth factors (IGFs) and vascular endothelial growth factor (VEGF) in diabetic kidney disease. Curr. Pharm. Des. 10, 3385-3394.
Godin, R.E., Robertson, E.J., Dudley, A.T., 1999. Role of BMP family members during kidney development. Int. J. Dev. Biol. 43, 405-411.
Hass, R., Kasper, C., Bohm, S., Jacobs, R., 2011. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 9, 12.
Hsu, R.K., Hsu, C.Y., 2016. The role of acute kidney injury in chronic kidney disease. Semin. Nephrol. 36, 283-292.
Kim, K.M., Oh, H.J., Choi, H.Y., Lee, H., Ryu, D.R., 2019. Impact of chronic kidney disease on mortality: A nationwide cohort study. Kidney Res. Clin. Pract. 38, 382-390.
Lee, Y., et, al., 2017. Characterization and multilineage differentiation of feline amniotic mesenchymal stem cells. Tissue Engineering and Regenerative Medicine 14, 681-692.
Lindstrom, N.O., Hohenstein, P., Davies, J.A., 2013. Nephrons require Rho-kinase for proximal-distal polarity development. Sci. Rep. 3, 2692.
Loomans, H.A., Andl, C.D., 2014. Intertwining of activin A and TGFbeta signaling: dual roles in cancer progression and cancer cell invasion. Cancers (Basel). 7, 70-91.
Lukomska, B., Stanaszek, L., Zuba-Surma, E., Legosz, P., Sarzynska, S., Drela, K., 2019. Challenges and controversies in human mesenchymal stem cell therapy. Stem. Cells. Int. 2019, 9628536.
Noh, S.A., Kim, T., Ju, J., 2021. Case reports of amniotic membrane derived-cell treatment for feline chronic renal failure. J. Anim. Reprod. Biotechnol. 36, 116-120.
Perini-Perera, S., Del-Angel-Caraza, J., Perez-Sanchez, A.P., Quijano-Hernandez, I.A., Recillas-Morales, S., 2021. Evaluation of chronic kidney disease progression in dogs with therapeutic management of risk factors. Front. Vet. Sci. 8, 621084.
Piyarungsri, K., Tangtrongsup, S., Thongtharb, A., Sodarat, C., Bussayapalakorn, K., 2020. The risk factors of having infected feline leukemia virus or feline immunodeficiency virus for feline naturally occurring chronic kidney disease. Vet. Integr. Sci. 18, 119-131.
Primeaux, M., Gowrikumar, S., Dhawan, P., 2022. Role of CD44 isoforms in epithelial-mesenchymal plasticity and metastasis. Clin. Exp. Metastasis. 39, 391-406.
Ramakrishnan, A., Torok-Storb B Fau - Pillai, M.M., Pillai, M.M., 2014. Primary marrow-derived stromal cells: isolation and manipulation. Methods Mol. Biol. 1035, 75–101.
Rashid, U., Yousaf, A., Yaqoob, M., Saba, E., Moaeen-Ud-Din, M., Waseem, S., Becker, S.K., Sponder, G., Aschenbach, J.R., Sandhu, M.A., 2021. Characterization and differentiation potential of mesenchymal stem cells isolated from multiple canine adipose tissue sources. BMC Vet. Res. 17, 388.
Singaravelu, K., Padanilam, B.J., 2009. In vitro differentiation of MSC into cells with a renal tubular epithelial-like phenotype. Ren. Fail. 31, 492-502.
Sobhani, A., Khanlarkhani, N., Baazm, M., Mohammadzadeh, F., Najafi, A., Mehdinejadiani, S., Sargolzaei Aval, F., 2017. Multipotent stem cell and current application. Acta Med. Iran. 55, 6-23.
Thomson, A.L., Berent, A.C., Weisse, C., Langston, C.E., 2019. Intra-arterial renal infusion of autologous mesenchymal stem cells for treatment of chronic kidney disease in cats: Phase I clinical trial. J. Vet. Intern. Med. 33, 1353-1361.
Thornton, C., 2017. Supporting quality of life in feline patients with chronic kidney disease. Vet. Nurs. 8, 200-206.
Tsujimura, T., Idei, M., Yoshikawa, M., Takase, O., Hishikawa, K., 2016. Roles and regulation of bone morphogenetic protein-7 in kidney development and diseases. World J. Stem. Cells. 8, 288-296.
Tucker, D., Still, K., Blom, A., Hollander, A., Kafienah, W., 2020. Over-confluence of expanded bone marrow mesenchymal stem cells ameliorates their chondrogenic capacity in 3D cartilage tissue engineering. Available online: https://www.biorxiv.org/content/10.1101/2020.01.08.897645v1.full
Vidane, A.S., Souza, A.F., Sampaio, R.V., Bressan, F.F., Pieri, N.C., Martins, D.S., Meirelles, F.V., Miglino, M.A., Ambrosio, C.E., 2014. Cat amniotic membrane multipotent cells are nontumorigenic and are safe for use in cell transplantation. Stem Cells Cloning. Adv. Appl. 7, 71-78.
Westenfelder, C., Togel, F.E., 2011. Protective actions of administered mesenchymal stem cells in acute kidney injury: relevance to clinical trials. Kidney Int. Suppl. 1, 103-106.
Xu, Y., Wang, Y.Q., Wang, A.T., Yu, C.Y., Luo, Y., Liu, R.M., Zhao, Y.J., Xiao, J.H., 2020. Effect of CD44 on differentiation of human amniotic mesenchymal stem cells into chondrocytes via Smad and ERK signaling pathways. Mol. Med. Rep. 21, 2357-2366.
Yang, H., Chen, J., Li, J., 2023. Isolation, culture, and delivery considerations for the use of mesenchymal stem cells in potential therapies for acute liver failure. Front. Immunol. 14, 1243220.
Zacharias, S., Welty, M.B., Sand, T.T., Black, L.L., 2021. Impact of allogeneic feline uterine-derived mesenchymal stromal cell intravenous treatment on renal function of nephrectomized cats with chronic kidney disease. Res. Vet. Sci. 141, 33-41.
Zhan, X.S., El-Ashram, S., Luo, D.Z., Luo, H.N., Wang, B.Y., Chen, S.F., Bai, Y.S., Chen, Z.S., Liu, C.Y., Ji, H.Q., 2019. A comparative study of biological characteristics and transcriptome profiles of mesenchymal stem cells from different canine tissues. Int. J. Mol. Sci. 20, 1485.