Geographical distribution and spatiotemporal clusters of African horse sickness outbreaks in Thailand https://doi.org/10.12982/VIS.2026.022
Main Article Content
Abstract
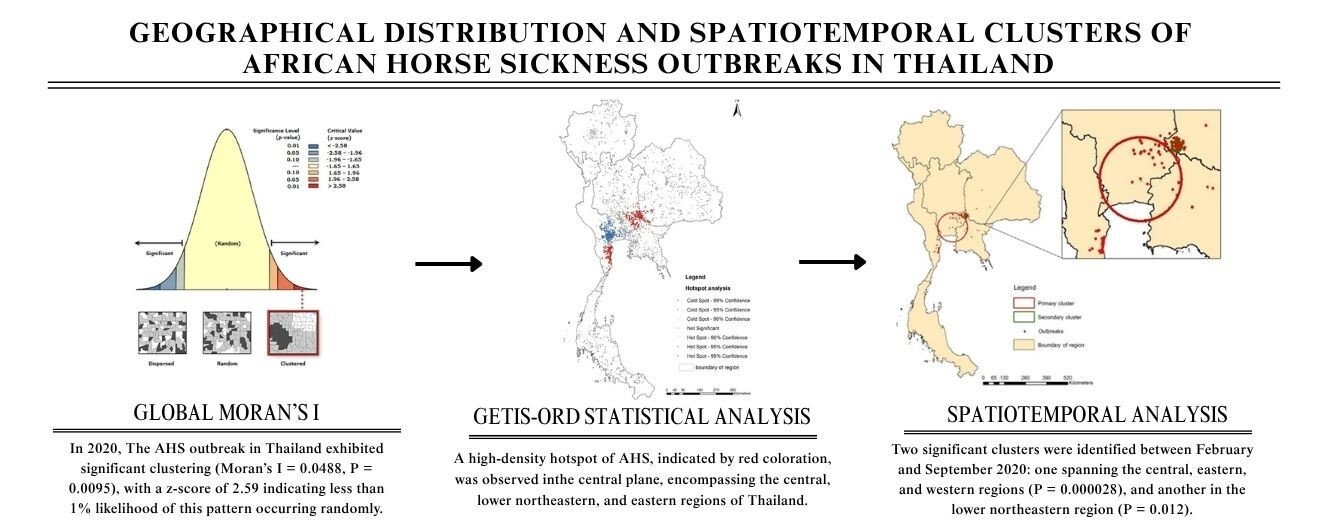
African horse sickness (AHS) is a deadly infectious vector-borne disease affecting equine species. Outbreaks of the disease can cause substantial economic loss due to its high mortality rate and the virus’s ability to extend beyond endemic areas. In March 2020, Thailand experienced the first confirmed AHS case, resulting in more than 600 horses dying and the mortality rate exceeding 90%. This study aims to determine the spatial distribution of AHS in Thailand. Initially, records on the first outbreak of AHS in 2020 were used for geoprocessing and visualized distribution. Subsequently, spatial and spatial-temporal statistical analyses were performed using QGIS and SaTScan 10.1 software. The results reveal the occurrence of AHS incidents in the central, lower northeastern, eastern, and western regions of Thailand at a total of 131 locations. The spatial analysis demonstrates significant clustering of AHS in 2020. Additionally, the Getis-Ord statistic reveals a high-density (hotspot) of AHS at the central plane, encompassing the central, lower northeastern, and eastern regions of Thailand. The space-time permutation model depicts the spatiotemporal pattern of AHS. The output identifies two significant clusters in the central part of the country, covering the central, eastern, and western regions (P-value 0.000028) and another cluster in the lower northeastern region (P-value 0.012) between February and September 2020. These findings provide crucial insights into the spatial and spatiotemporal distribution of AHS in Thailand, which is necessary for improving disease management and prevention strategies.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Publishing an article with open access in Veterinary Integrative Sciences leaves the copyright with the author. The article is published under the Creative Commons Attribution License 4.0 (CC-BY 4.0), which allows users to read, copy, distribute and make derivative works from the material, as long as the author of the original work is cited.
References
Carpenter, S., Mellor, P.S., Fall, A.G., Garros, C., Venter, G.J., 2017. African horse sickness virus: history, transmission, and current status. Annu. Rev. Entomol. 62, 343-358.
Castillo‐Olivares, J., 2020. African horse sickness in Thailand: challenges of controlling an outbreak by vaccination. Equine Vet. J. 53(1), 9.
Chimera, E.T., Fosgate, G.T., Etter, E.M.C., Jemberu, W.T., Kamwendo, G., Njoka, P., 2022. Spatio-temporal patterns and risk factors of foot-and-mouth disease in malawi between 1957 and 2019. Prev. Vet. Med. 204, 105639.
Dennis, S.J., Meyers, A.E., Hitzeroth, II, Rybicki, E.P., 2019. African horse sickness: a review of current understanding and vaccine development. Viruses. 11(9), 844.
Diouf, N.D., Etter, E., Lo, M.M., Lo, M., Akakpo, A.J., 2013. Outbreaks of african horse sickness in senegal, and methods of control of the 2007 epidemic. Vet. Rec. 172(6), 152-152.
Fairbanks, E.L., Baylis, M., Daly, J.M., Tildesley, M.J., 2022. Inference for a spatio-temporal model with partial spatial data: African horse sickness virus in Morocco. Epidemics. 39, 100566.
Faverjon, C., Leblond, A., Hendrikx, P., Balenghien, T., de Vos, C.J., Fischer, E.A.J., de Koeijer, A.A., 2015. A spatiotemporal model to assess the introduction risk of african horse sickness by import of animals and vectors in france. BMC Vet. Res. 11(1), 127.
Graham, A.J., Peter, M.A., Danson, F.M., 2004. Spatial analysis for epidemiology. Acta. Trop. 91(3), 219-225.
Grewar, J.D., Koen, P., Davey, S., Visser, D., Russouw, E., Buhrmann, G., Weyer, C.T., Guthrie, A.J., Quan, M., 2013. The 2011 outbreak of African horse sickness in the African horse sickness controlled area in South Africa: Original research. J. S. Afr. Vet. Assoc. 84(1), 1-7.
Howell, P.G., 1960. The 1960 epizootic of African horse sickness in the middle east and S.W. Asia. J. S. Afr. Vet. Assoc. 31(3), 329-334.
Kim, K., Xu, T., Kannan Villalan, A., Chi, T., Yu, X., Jin, M., Wu, R., Ni, G., Sui, S., Wang, Z., Wang, X., 2024. Environmental and historical determinants of African horse sickness: Insights from predictive modeling. Transbound. Emerg. Dis. 2024, 5586647.
Koch, T., 2012. Knowing its place: mapping as medical investigation. Lancet. 379(9819), 887-888.
Kulldorff, M., 2018. Satscantm user guide. Available online: https://www.satscan.org/
Li, Y., An, Q., Sun, Z., Gao, X., Wang, H., 2023. Risk factors and spatiotemporal distribution of lumpy skin disease occurrence in the asian continent during 2012–2022: an ecological niche model. Transbound. Emerg. Dis. 2023(1), 6207149.
López, M.S., Müller, G.V., Sione, W.F., 2018. Analysis of the spatial distribution of scientific publications regarding vector-borne diseases related to climate variability in South America. Spat. Spatio-Temporal Epidemiol. 26, 35-93.
Mclafferty, S., 2015. Disease cluster detection methods: recent developments and public health implications. Ann. GIS. 21(2), 127-133.
Palaniyandi, M., Anand, P., Maniyosai, R., 2014. Spatial cognition: a geospatial analysis of vector borne disease transmission and the environment, using remote sensing and GIS. Int. J. Mosq. 1(3), 39-54.
Portas, M., Boinas, F., Sousa, J.O.E., Rawlings, P., 1999. African horse sickness in portugal: a successful eradication programme. Epidemiol. Infect. 123(2), 337-346.
Punyapornwithaya, V., Seesupa, S., Phuykhamsingha, S., Arjkumpa, O., Sansamur, C., Jarassaeng, C., 2022. Spatio-temporal patterns of lumpy skin disease outbreaks in dairy farms in Northeastern Thailand. Front. Vet. Sci. 9, 957306.
Robin, M., Page, P., Archer, D., Baylis, M., 2016. African horse sickness: the potential for an outbreak in disease‐free regions and current disease control and elimination techniques. Equine Vet. J. 48(5), 659-669.
Rodriguez, M., Hooghuis, H., Castaño, M., 1992. African horse sickness in Spain. Vet. Microbiol. 33(1), 129-142.
Toh, X., Wang, Y., Rajapakse, M.P., Lee, B., Songkasupa, T., Suwankitwat, N., Kamlangdee, A., Judith Fernandez, C., Huangfu, T., 2022. Use of nanopore sequencing to characterize African horse sickness virus (AHSV) from the African horse sickness outbreak in Thailand in 2020. Transbound. Emerg. Dis. 69(3), 1010-1019.
Waldhör, T., 1996. The spatial autocorrelation coefficient moran’s i under heteroscedasticity. Stat. Med. 15(7-9), 887-892.
Wibowo, S.E., Andityas, M., Nuraini, D.M., Zurbein, Nugraheni, Y.R., Awaludin, A., Rahayu, P., Insulistyowati, A., 2024. Spatial analysis of bovine anaplasmosis in Jambi province, Indonesia: 2018-2022. Vet. Integr. Sci. 22(3), 857–869.
World Organisation for Animal Health (OIE), 2020. Guidelines on preparedness and implementation of emergency vaccination in the asian region. Paris, France, OIE.