Effect of Lactiplantibacillus plantarum with vegetable oil supplementation on rumen fermentation and lactation performance in dairy goats https://doi.org/10.12982/VIS.2026.053
Main Article Content
Abstract
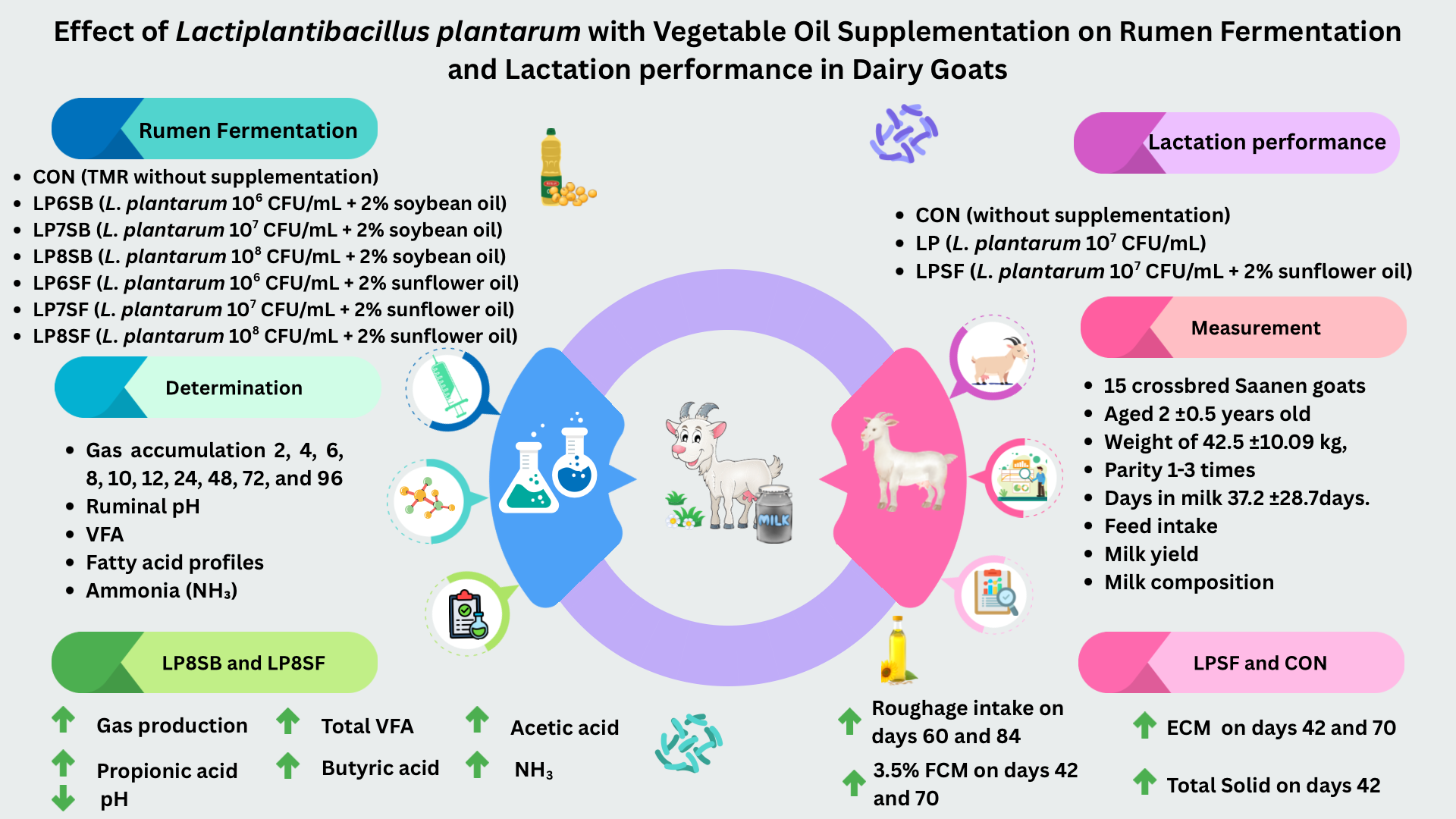
This study was conducted to determine the effect of different concentrations of Lactiplantibacillus plantarum with vegetable oils on in vitro rumen fermentation and lactation performance in dairy goats. The in vitro rumen fermentation was divided into seven groups: CON (TMR without supplementation), LP6SB (TMR + 106 CFU/mL of L. plantarum + 2% soybean oil), LP7SB (TMR + 107 CFU/mL of L. plantarum + 2% soybean oil), LP8SB (TMR + 108 CFU/mL of L. plantarum + 2% soybean oil), LP6SF (TMR + 106 CFU/mL of L. plantarum + 2% sunflower oil), LP7SF (TMR + 107 CFU/mL of L. plantarum + 2% sunflower oil), and LP8SF (TMR + 108 CFU/mL of L. plantarum + 2% sunflower oil). The rumen fermentation parameters measured included ruminal pH, fatty acid profiles, and ammonia (NH₃) concentrations. The lactation performance experiment was divided into three groups: CON (without supplementation), LP (L. plantarum 70 mL of 109 CFU/mL/head), and LPSF (L. plantarum 70 mL of 109 CFU/mL/head + 2% sunflower oil). The performance parameters were feed intake, milk yield, and milk composition. In vitro gas production at 24 hours showed that LP8SB and LP8SF were the highest among other groups (58.87 mL and 59.30 mL) (P<0.001). However, ruminal pH at 24 hours for LP8SB and LP8SF was the lowest compared with other groups (6.74) (P<0.001). The LP8SB and LP8SF exhibited the highest acetic acid, propionic acid, butyric acid, and total VFA production (87.60 mmol/L and 79.51 mmol/L, respectively). The NH3 levels at 24 hours revealed that LP8SB and LP8SF were the highest concentrations compared with other groups (24.13 and 24.08 mM, respectively) (P < 0.001). Roughage intake on days 60 and 84 for LPSF was higher than for LP (950 and 1000 vs. 820 and 890 g/d, respectively) but not different with control group. Milk fat content, milk protein content, lactose, and solid not fat content did not show significant differences among treatments (P > 0.05). It may be concluded that L. plantarum 108 CFU/mL with both oil supplementations increased rumen fermentation concentrations while L. plantarum 107 CFU/mL with sunflower oil supplementation increased roughage intake but milk composition were not affected by L. plantarum 107 CFU/mL with sunflower oil supplementation
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Publishing an article with open access in Veterinary Integrative Sciences leaves the copyright with the author. The article is published under the Creative Commons Attribution License 4.0 (CC-BY 4.0), which allows users to read, copy, distribute and make derivative works from the material, as long as the author of the original work is cited.
References
Abo El-Nor, S.A.H., Khattab, M.S.A., 2012. Enrichment of milk with conjugated linoleic acid by supplementing diets with fish and sunflower oil. Pak. J. Biol. Sci. 15(14), 690–693.
Alazzeh, A.Y., Sultana, H., Beauchemin, K.A., Wang, Y., Holo, H., Harstad, O.M., McAllister, T.A., 2013. Using strains of propionibacteria to mitigate methane emissions in vitro. Acta Agric. Scand. A Anim. Sci. 62(4), 263–272.
Anzhany, D., Toharmat, T., Despal, D., 2023. Nutrient and fatty acid composition of elephant and king grasses from different altitudes. IOP Conf. Ser. Earth Environ. Sci. 1183, 012001.
AOAC, 1990. Official Methods of Analysis, 15th edition. Association of Official Analytical Chemists, Washington, DC.
Arawolo, M.A., He, J. 2018. Use of probiotics and botanical extracts to improve ruminant production in the tropics: a review. Anim. Nutr. 4(3), 241–249.
Aschenbach, J.R., Zebeli, Q., Patra, A.K., Greco, G., Amasheh, S., Penner, G.B., 1966. Symposium review: the importance of the ruminal epithelial barrier for a healthy and productive cow. J. Dairy. Sci. 102, 1866–1882.
Astuti, W.D., Wiryawan, K.G., Wina, E., Widyastuti, Y., Suharti, S., Ridwan, R., 2018. Effects of selected Lactobacillus plantarum as probiotic on in vitro ruminal fermentation and microbial population. Pak. J. Nutr. 17(3), 131–139.
Astuti, W.D., Ridwan, R., Fidriyanto, R., Rohmatussolihat, R., Sari, N.F., Sarwono, K.A., Fitri, A., Widyastuti, Y., 2022. Changes in rumen fermentation and bacterial profiles after administering Lactiplantibacillus plantarum as a probiotic. Vet. World. 15(8), 1969-1974.
Blummel, M., Steingaβ, H., Becker, K., 1997. The relationship between in vitro gas production, in vitro microbial biomass yield and 15 N incorporation and its implications for the prediction of voluntary feed intake of roughages. Br. J. Nutr. 77(6), 911–921.
Bouattour, M.A., Casal, R., Albanell, R., Such, X., Caja, G., 2008. Feeding soybean oil to dairy goats increases conjugated linoleic acid in milk. J. Dairy Sci. 91, 2399–2407.
Chaney, A.L., Marbach, E.P., 1992. Modified reagents for determination of urea and ammonia. Clin. Chem. 8, 130–132.
Christie, W.W., 1981. The effect of diet and other factors on the lipid composition of ruminant tissues and milk. In: Christie, W.W. (Ed.), Lipid Metabolism in Ruminant Animals. Pergamon Press, Oxford, pp. 193–226.
Contreras-Govea, F.E., Muck, R.E., Broderick, G.A., Weimer, P.J., 2013. Lactobacillus plantarum effects on silage fermentation and in vitro microbial yield. Anim. Feed Sci. Tech. 179, 61–68.
DePeters, E., German, J., Taylor, S., Essex, S., Perez-Monti, H., 2001. Fatty acid and triglyceride composition of milk fat from lactating Holstein cows in response to supplemental canola oil. J. Dairy Sci. 84, 929–936.
Geisseler, D., Horwath, W.R., Joergensen, R.G., Ludwig, B., 2011. Pathways of Nitrogen Utilization by Soil Microorganisms– A Review. Soil Biol. Biochem. 42, 2058–2067.
Ghorban, K., Knox, K., Ward, G., 1966. Concentrations of volatile fatty acids and lactic acid in the rumen as influenced by diet and post-feeding time. J. Dairy Sci. 49, 1515–1518.
Goering, H.K., Van Soest, P.J., 1970. Forage fiber analysis: apparatus, reagents, pocedures and some applications. United States Department of Agriculture, Washington DC.
Groehn, J.A., Kaneene, J.B., Foster, D., 1992. Risk factors associated with lameness in lactating dairy cattle in Michigan. Prev. Vet. Med. 14, 77–85.
Guo, G., Shen, C., Liu, Q., Zhang, S., Shao, T., Wang, C., Wang, Y., Xu, Q., Huo, W., 2020. The effect of lactic acid bacteria inoculums on in vitro rumen fermentation, methane production, ruminal cellulolytic bacteria populations and cellulase activities of corn stover silage. J. Integr. Agric. 19(3), 838–847.
Holm, S., 1979. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6, 65–70.
Izuddin, W.I., Loh, T.C., Samsudin, A.A., Foo, H.L., 2018. In vitro study of postbiotics from Lactobacillus plantarum RG14 on rumen fermentation and microbial population. R. Bras. Zootec. 47, e20170255.
Jiao, P.X., Liu, F.Z., Beauchemin, K.A., Yang, W.Z., 2017. Impact of strain and dose of lactic acid bacteria on in vitro ruminal fer-mentation with varying media pH levels and feed substrates. Anim. Feed Sci. Technol. 224(2), 1–13.
Karami, M., Ponnampalam, E.N., Hopkins, D.L., 2013. The effect of palm oil or canola oil on feedlot performance, plasma and tissue fatty acid profile and meat quality in goats. Meat Sci. 94, 165-169.
Kenney, N.M., Vanzant, E.S., Harmon, D.L., McLeod, K.R., 2015. Direct-fed microbials containing lactate-producing bacteria in-fluence ruminal fermentation but not lactate utilization in steers fed a high-concentrate diet. J. Anim. Sci. 93(5), 2336–2348.
Krnjai´, S., Cincovi´, M., Djokovi´, R., Beli´, B., Ježek, J., Stariˇ, J., 2022. The influence of energy balance lipolysis and ketogenesis on metabolic adaptation in cows milked twice and three times daily. Metabolites. 12, 1090.
Lounglawan, P., Suksombat, W., 2001. Effect of soybean oil and lactic acid bacteria supplementation on performance and CLA accumulation in milk of dairy cows. J. Anim. Vet. Adv. 10(7), 868-874.
McCann, J.C., Luan, S., Cardoso, F.C., Derakhshani, H., Khafipour, E., Loor, J.J., 2016. Induction of subacute ruminal acidosis affects the ruminal microbiome and epithelium. Front. Microbiol. 7, 701.
McDonald, P., Edwards, R.A., Greenhalgh, J.F.D., Morgan, C.A., Sinclair, L.A., Wilkinson, R.G., 2010. Animal Nutrition, 7th edition. Prentice Hall, New Jersey.
Menke, K.H., Raab, L., Salewski, A., Steingaß, H., Fritz, D., Schneider, W., 1979. The estimation of the digestibility and metabolizable energy content of ruminant feeding stuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 93, 217–222.
Menke, K., Steingass, H., 1988. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 28, 7-55.
Monteiro, H.F., Paula, E.M., Muck, R.E., Broderick, G.A., Faciola1, A.P., 2021. Effects of lactic acid bacteria in a silage inoculant on ruminal nutrient digestibility, nitrogen metabolism, and lactation performance of high-producing dairy cows. J. Dairy Sci. 104, 8826–8834.
Morand-Fehr, P., Sauvant, D., 1978. Nutrition and optimum performance of dairy goats. Livest. Prod. Sci. 5, 203-213.
Muck, R.E., Filya, I., Contreras-Govea, F.E., 2007. Inoculant effects on alfalfa silage: in vitro gas and volatile fatty acid production. J. Dairy Sci. 90(11), 5115–5125.
Nalla, K., Manda, N.K., Dhillon, H.S., Kanade, S.R., Rokana, N., Hess, M., Puniya, A.K., 2022. Impact of probiotics on dairy production efficiency. Front. Microbiol. 13, 805963.
National Research Council, 2001. Nutrient Requirements of Dairy Cattle, 7th edition. National Academy Press, Washington, DC.
Nocek, J.E., Kautz, W.P., Leedle, J.A.Z., Allman, J.G., 2002. Ruminal supplementation of direct-fed microbials on diurnal pH variation and in situ digestion in dairy cattle. J. Dairy Sci. 85(2), 429–433.
O’Fallon, J.V., Busboom, J.R., Nelson, M.L., Gaskins, C.T., 2007. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils and feedstuffs. J. Anim. Sci. 85, 1511 – 1521.
Oliveira, A.S., Weinberg, Z.G., Ogunade, I.M., Cervantes, A.A.P., Arriola, K.G., Jiang, Y., Kim, D., Li, X., Gonçalves, M.C.M., Vyas, D., Adesogan, A.T., 2017. Meta-analysis of effects of inoculation with homofermentative and facultative heterofermentative lactic acid bacteria on silage fermentation, aerobic stability, and the performance of dairy cows. J. Dairy Sci. 100, 4587–4603.
Ørskov, E., McDonald, I., 1979. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 92(2), 499-503.
Oskoueian, E., Jahromi, M.F., Jafari, S., Shakeri, M., Le, H.H., Ebrahimi, M., 2021. Manipulation of rice straw silage fermentation with different types of lactic acid bacteria inoculant affects rumen microbial fermentation characteristics and methane production. Vet. Sci. 8(6), 100.
Palmquist, D., Beaulieu, A., Barbano, D., 1993. Feed and animal factors influencing milk fat composition. J. Dairy Sci. 76, 1753–1771.
Ridwan, R., Bungsu, W.A., Astuti, W.D., Rohmatussolihat, R., Sari, N.F., Fidriyanto, R., Jayanegara, A., Wijayanti, I., Widyastuti, Y., 2018. The use of lactic acid bacteria as ruminant probiotic candidates based on in vitro rumen fermentation characteristics. Bull. Peternak. 42(1), 31–36.
Silva, T.M., Oliveira, R.O., Barbosa, L.P., Garcez, A.F., Bagaldo, A.R., 2011. Preliminarystudy onmeat quality of goats fed levels of licury oil in the diet. Asian-Australas. J. Anim. Sci. 24, 1112–1119.
Soriano, A.P., Mamuad, L.L., Kim, S.H., Choi, Y.J., Jeong, C.D., 2014. Effect of Lactobacillus mucosae on in vitro rumen fermentation characteristics of dried brewers grain, methane production and bacterial diversity. Asian-Australas. J. Anim. Sci. 27(11), 1562–1570.