Bacillus velezensis from the Muscovy Duck gut: A potential probiotic for controlling poultry pathogens https://doi.org/10.12982/VIS.2026.060
Main Article Content
Abstract
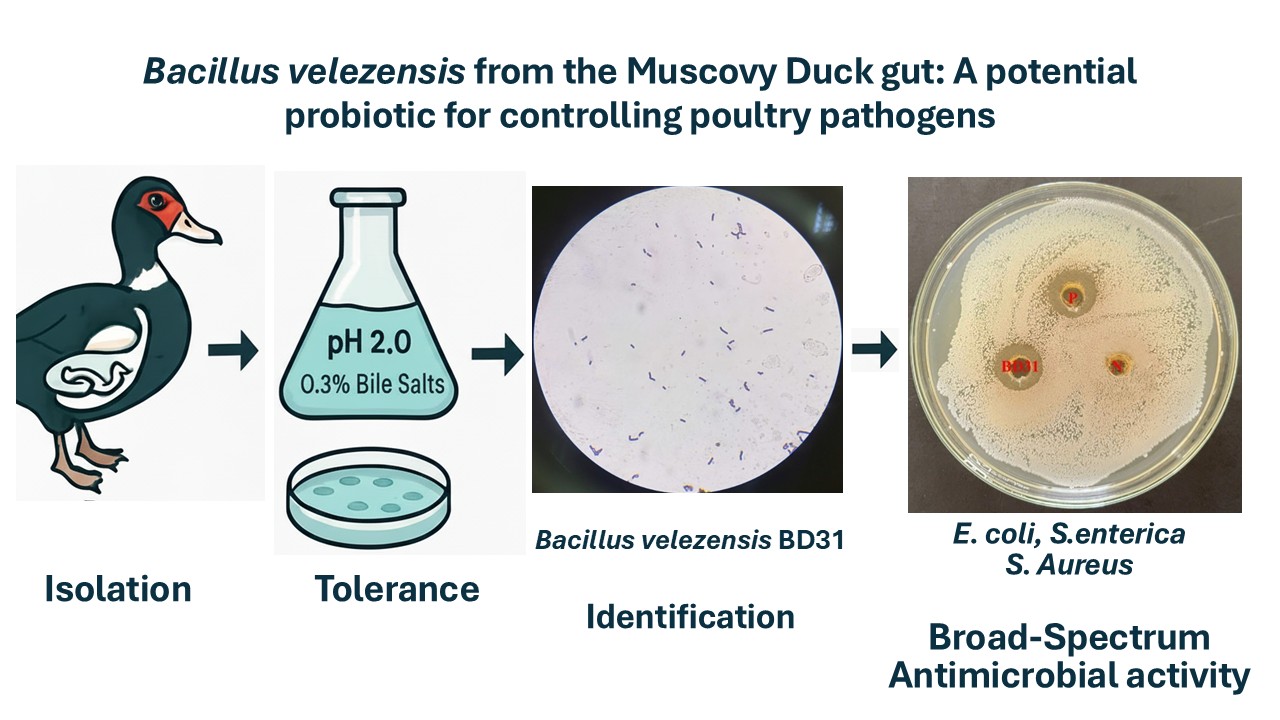
Bacterial diseases and antimicrobial resistance threaten the poultry industry, necessitating antibiotic alternatives. Probiotics, especially Bacillus species, are a promising solution. This study explored the gut of the Muscovy duck (Cairina moschata (Linnaeus, 1758)) as an untapped source for novel probiotic candidates. The objectives were to isolate native Bacillus strains from Muscovy duck guts, identify the most promising candidate, and characterize its probiotic potential by assessing its resilience and antimicrobial activity against key poultry pathogens. A total of 40 bacterial strains were isolated from the gut contents of 50 Muscovy ducks using a heat-shock enrichment method. All isolates were screened in vitro for their tolerance to simulated gastrointestinal conditions (pH 2.0 and 0.3% bile salts). The antimicrobial activity of their cell-free supernatants was assessed against Escherichia coli, Salmonella enterica, and Staphylococcus aureus using the agar well diffusion method. The most potent isolate was definitively identified by 16S rRNA gene sequencing. Isolate BD31 demonstrated exceptional tolerance to both acidic and bile salt conditions. Furthermore, it exhibited potent, broad-spectrum antagonistic activity, producing significant inhibition zones against E. coli (12.5 mm), S. enterica (11.8 mm), and S. aureus (8.5 mm). Molecular analysis identified this superior isolate as Bacillus velezensis with 99.76% sequence identity to the type strain. The Muscovy duck gut is a valuable source for novel probiotics. Bacillus velezensis BD31 displays superior gastrointestinal tolerance and potent antimicrobial activity, positioning it as an excellent candidate for a direct-fed microbial to control poultry pathogens. In vivo validation is warranted.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
Publishing an article with open access in Veterinary Integrative Sciences leaves the copyright with the author. The article is published under the Creative Commons Attribution License 4.0 (CC-BY 4.0), which allows users to read, copy, distribute and make derivative works from the material, as long as the author of the original work is cited.
References
AbdelRahman, M.A.A., Amer, F., 2021. Characterization of toxin gene profiles and antibiotic resistance genes of methicillin resistant Staphylococcus aureus isolated from ducks. Adv. Anim. Vet. Sci. 9(8), 1150-1158.
Afroj, S., Brannen, A.D., Nasrin, S., Al Mouslem, A., Hathcock, T., Maxwell, H., Rasmussen-Ivey, C.R., Sandage, M.J., Davis, E.W., Panizzi, P., 2021. Bacillus velezensis AP183 inhibits Staphylococcus aureus biofilm formation and proliferation in murine and bovine disease models. Front. Microbiol. 12, 746410.
Aguidissou, O.N.C., Akpo, Y., Adoko, A.M.J., Adoligbè, C.M., Koutinhoui, B.G., Boko, C.K., Farougou, S., 2022. Avian Salmonellosis and Colibacillosis: Risk factors, antibiotic resistance, public health impact and biological control. Int. J. Poult. Sci. 21(3), 90-106.
Al Azad, S., Moazzem Hossain, K., Rahman, S.M.M., Al Mazid, M.F., Barai, P., Gazi, M.S., 2020. In ovo inoculation of duck embryos with different strains of Bacillus cereus to analyse their synergistic post-hatch anti-allergic potentialities. Vet. Med. Sci. 6(4), 992-999.
Arya, M., Shahi, N., Bisht, I., Pandey, N., Mallik, S.K., 2025. Probiotic potential of Bacillus velezensis STPB10 sourced from the gut microbiota of a hillstream fish Schizothorax richardsonii (Gray, 1832) for aquaculture applications. Sci. Rep. 15(1), 17580.
Barzegar, H., Alizadeh Behbahani, B., Falah, F., 2021. Safety, probiotic properties, antimicrobial activity, and technological performance of Lactobacillus strains isolated from Iranian raw milk cheeses. Food Sci. Nutr. 9(8), 4094-4107.
Brutscher, L.M., Gebrechristos, S., Garvey, S.M., Spears, J.L., 2024. Genetic and phenotypic characterization of Bacillus velezensis strain BV379 for human probiotic applications. Microorganisms. 12(3), 436.
da Rosa, C.E., Pinilla, C.M.B., Toss, L.D., Brandelli, A., 2025. In silico and in vitro characterization of Bacillus velezensis P45: Screening for a novel probiotic candidate. Foods. 14(13), 2334.
De, S., Kaur, G., Roy, A., Dogra, G., Kaushik, R., Yadav, P., Singh, R., Datta, T.K., Goswami, S.L., 2010. A simple method for the efficient isolation of genomic DNA from Lactobacilli isolated from traditional Indian fermented milk (dahi). Indian J. Microbiol. 50, 412-418.
Golnari, M., Bahrami, N., Milanian, Z., Rabbani Khorasgani, M., Asadollahi, M.A., Shafiei, R., Fatemi, S.S.A., 2024. Isolation and characterization of novel Bacillus strains with superior probiotic potential: comparative analysis and safety evaluation. Sci. Rep. 14(1), 1457.
Hamed, R.I., Nabil, N.M., Tawakol, M.M., AbouKhadra, S.H., 2024. An overview of current status of Salmonellosis in duck farms in Egypt. Egypt. J. Anim. Health. 4(3), 182-189.
Hanim, C., Cahya, V.A., Yusiati, L.M., Kurniawati, A., 2021. Isolation and identification of bacteriocin-producing Bacillus strain isolated from the gastrointestinal tract of Indonesian native chicken (Gallus domesticus). In Proceedings of the 10th International Seminar and 12th Congress of Indonesian Society for Microbiology (ISISM 2019), Surakarta, Indonesia, 29-30 August 2019, pp. 32-36.
Hellany, H., Assaf, J.C., Barada, S., el-Badan, D., Hajj, R. El, Abou Najem, S., Abou Fayad, A.G., Khalil, M.I., 2024. Isolation and characterization of Bacillus subtilis BSP1 from soil: antimicrobial activity and optimization of fermentation conditions. Processes. 12(8), 1621.
Hosseini, N.G., Modarressi, M.H., Mousavi, S.N., Ebrahimi, M.T., 2018. Evaluation of novel probiotic Bacillus strains based on enzyme production and protective activity against salmonellosis. J. Hell. Vet. Med. Soc. 69(4), 1205-1212.
Hu, J., Chen, L., Li, G., Pan, Y., Lu, Y., Chen, J., Xiong, W., Zeng, Z., 2023. Prevalence and genetic characteristics of fosB-positive Staphylococcus aureus in duck farms in Guangdong, China in 2020. J. Antimicrob. Chemother. 78(3), 802-809.
Husna, Kim, B.E., Won, M.H., Jeong, M.I., Oh, K.K., Park, D.S., 2023. Characterization and genomic insight of surfactin-producing Bacillus velezensis and its biocontrol potential against pathogenic contamination in lettuce hydroponics. Environ. Sci. Pollut. Res. 30(58), 121487-121500.
Khushboo, Karnwal, A., Malik, T., 2023. Characterization and selection of probiotic lactic acid bacteria from different dietary sources for development of functional foods. Front. Microbiol. 14, 1170725.
Krysiak, K., Konkol, D., Korczyński, M., 2021. Overview of the use of probiotics in poultry production. Animals. 11(6), 1620.
Latorre, J.D., Hernandez-Velasco, X., Wolfenden, R.E., Vicente, J.L., Wolfenden, A.D., Menconi, A., Bielke, L.R., Hargis, B.M., Tellez, G., 2016. Evaluation and selection of Bacillus species based on enzyme production, antimicrobial activity, and biofilm synthesis as direct-fed microbial candidates for poultry. Front. Vet. Sci. 3, 95.
Li, X., Li, W., Zhao, L., Li, Y., He, W., Ding, K., Cao, P., 2024. Characterization and assessment of native lactic acid bacteria from broiler intestines for potential probiotic properties. Microorganisms. 12(4), 749.
Liu, G., Kong, Y., Fan, Y., Geng, C., Peng, D., Sun, M., 2017. Whole-genome sequencing of Bacillus velezensis LS69, a strain with a broad inhibitory spectrum against pathogenic bacteria. J. Biotechnol. 249, 20-24.
Mazanko, M.S., Popov, I.V, Prazdnova, E.V, Refeld, A.G., Bren, A.B., Zelenkova, G.A., Chistyakov, V.A., Algburi, A., Weeks, R.M., Ermakov, A.M., 2022. Beneficial effects of spore-forming bacillus probiotic bacteria isolated from poultry microbiota on broilers’ health, growth performance, and immune system. Front. Vet. Sci. 9, 877360.
Menconi, A., Morgan, M.J., Pumford, N.R., Hargis, B.M., Tellez, G., 2013. Physiological properties and Salmonella growth inhibition of probiotic Bacillus strains isolated from environmental and poultry sources. Int. J. Bacteriol. 2013, 958408.
Naeem, M., Ahmed, I., Ahmed, S., Ahmed, Z., Riaz, M.N., Ghazanfar, S., 2018. Screening of cattle gut associated Bacillus strains for their potential use as animal probiotic. Indian J. Anim. Res. 53(10).
Nair, A.S., Dubhashi, A.V., 2016. In vitro transit tolerance of probiotic Bacillus species in human gastrointestinal tract. Int. J. Sci. Res. 5, 1899-1902.
Patil, S.S., Shinduja, R., Suresh, K.P., Phukan, S., Kumar, S., Sengupta, P.P., Amachawadi, R.G., Raut, A., Roy, P., Syed, A., 2021. A systematic review and meta-analysis on the prevalence of infectious diseases of ducks: a world perspective. Saudi J. Biol. Sci. 28(9), 5131-5144.
Penaloza-Vazquez, A., Ma, L.M., Rayas-Duarte, P., 2019. Isolation and characterization of Bacillus spp. strains as potential probiotics for poultry. Can. J. Microbiol. 65(10), 762-774.
Pérez-Sánchez, T., Mora-Sánchez, B., Balcázar, J.L., 2018. Biological approaches for disease control in aquaculture: advantages, limitations and challenges. Trends Microbiol. 26(11), 896-903.
Perini, H.F., de Barros Pereira, B., Sousa, E.G., Matos, B.S., da Silva Prado, L.C., de Carvalho Azevedo, V.A., de Castro Soares, S., da Silva, M.V., 2024. Inhibitory effect of Bacillus velezensis 1273 strain cell-free supernatant against developing and preformed biofilms of Staphylococcus aureus and MRSA. Microb. Pathog. 197, 107065.
Plaza-Diaz, J., Ruiz-Ojeda, F.J., Gil-Campos, M., Gil, A., 2019. Mechanisms of action of probiotics. Adv. Nutr. 10, S49-S66.
Rabbee, M.F., Ali, M.S., Choi, J., Hwang, B.S., Jeong, S.C., Baek, K., 2019. Bacillus velezensis: a valuable member of bioactive molecules within plant microbiomes. Molecules. 24(6), 1046.
Rahimoon, M.M., Mirani, A.H., Sahito, J.K., Bhutto, A.L., Khoso, P.A., Leghari, R.A., Kaka, A., Aqeel, M., Bughio, R., Kalwar, Q., 2023. Ameliorative effect of probiotics used in animals: a comprehensive review. Pure Appl. Biol. 13(2), 179-193.
Ramlucken, U., Lalloo, R., Roets, Y., Moonsamy, G., van Rensburg, C.J., Thantsha, M.S., 2020. Advantages of Bacillus-based probiotics in poultry production. Livest. Sci. 241, 104215.
Shen, C., Zhang, Y., 2022. Biochemistry test of bacteria-1 (urease test, carbohydrate fermentation, catalase test, oxidase test). In: Introductory microbiology lab skills and techniques in food science. Elsevier, Amsterdam, pp. 67-76.
Söylemez-Milli, N., Ertürkmen, P., Alp Baltakesmez, D., 2025. The resistance abilities of some Bacillus species to gastrointestinal tract conditions: Whole genome sequencing of the novel candidate probiotic strains Bacillus clausii BA8 and Bacillus subtilis BA11. Food Sci. Nutr. 13(2), e70018.
Su, T., Shen, B., Hu, X., Teng, Y., Weng, P., Wu, Z., Liu, L., 2024. Research advance of Bacillus velezensis: bioinformatics, characteristics, and applications. Food Sci. Hum. Wellness. 13(4), 1756-1766.
Swacita, I.B.N., Suardana, I.W., Sudisma, I.G.N., Wihadmadyatami, H., 2022. Molecular identification of Lactic acid bacteria SR6 strain and evaluation of its activity as an anticancer in T47D cell line. Vet. World. 15(6), 1583-1588.
Tamura, K., Stecher, G., Kumar, S., 2021. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 38(7), 3022-3027.
Tripathi, N., Sapra, A., 2020. Gram staining. StatPearls, Treasure Island, FL.
Truong, Q.P., Phan, T.C.T., Vu, H.H., Pham, T.T.N., Huynh, T.G., Vu, N.U., 2021. Isolation of potential probiotic Bacillus subtilis CM3. 1 and its effects on the water quality and growth performance of the whiteleg shrimp Litopenaeus vannamei in the Mekong Delta, Vietnam. AACL Bioflux. 14(6), 3347-3357.
Wang, L., Wang, H., Li, X., Zhu, M., Gao, D., Hu, D., Xiong, Z., Li, X., Qian, P., 2024. Bacillus velezensis HBXN2020 alleviates Salmonella Typhimurium infection in mice by improving intestinal barrier integrity and reducing inflammation. eLife. 13, RP93423.
Zhu La, A.L.T., Wen, Q., Xiao, Y., Hu, D., Liu, D., Guo, Y., Hu, Y., 2024. A New Bacillus velezensis strain CML532 improves chicken growth performance and reduces intestinal Clostridium perfringens colonization. Microorganisms. 12(4), 771.