Endocrine Dysfunction Induced by Immune Checkpoint Inhibitors
Keywords:
Immune checkpoint inhibitors, Endocrine dysfunction, immune-related adverse eventsAbstract
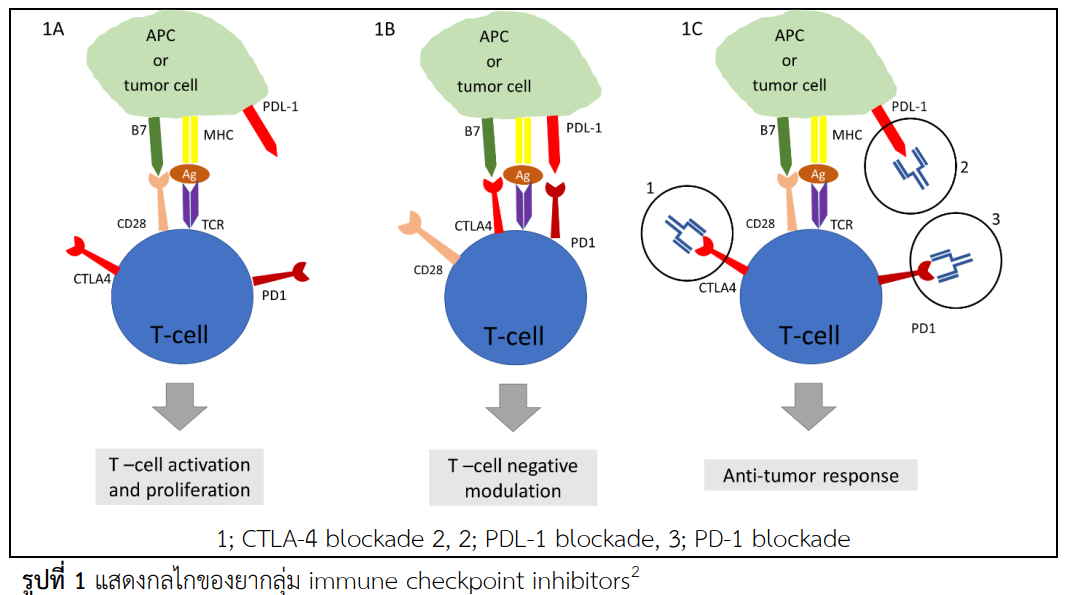
In the modern era of cancer treatment. it is constantly evolving with new breakthroughs and discoveries. Immune checkpoint inhibitors (ICPi) are a new and effective class of cancer immunotherapy. Several human monoclonal antibodies directed against immune checkpoints, including T lymphocyte antigen 4 and program cell death protein 1 has been implemented for cancer treatment in order to promote effector T cell response to tumors. Despite their antitumor activity, a significant number of patients demonstrated autoimmunity leading to immune-related adverse events (IRAEs). IRAEs can potentially affect the functions of multiple organs including the gastrointestinal tract, kidneys, nervous system, liver, eyes, skin, pancreas, and endocrine system. Many of which can be life-threatening. The spectrum of endocrine dysfunction experienced by patients treated with ICPi includes hypophysitis, thyroid dysfunction, diabetes mellitus, primary adrenal insufficiency, and hypoparathyroidism. This review summarizes the recent clinical studies and treatment guidelines for different IRAEs with a focus on the incidence, pathophysiology, clinical course, and treatment.
Downloads
References
2. Palmieri DJ, Carlino MS. Immune Checkpoint Inhibitor Toxicity. Curr Oncol Rep. 2018;20(9):72. doi:10.1007/s11912-018-0718-6
3. Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer immunotherapy - immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol. 2017;13(4):195-207. doi:10.1038/nrendo.2016.205
4. Cooper GM. The Development and Causes of Cancer. Cell Mol Approach 2nd Ed. 2000. https://www.ncbi.nlm.nih.gov/books/NBK9963/. Accessed December 26, 2019.
5. Girotra M, Hansen A, Farooki A, et al. The Current Understanding of the Endocrine Effects From Immune Checkpoint Inhibitors and Recommendations for Management. JNCI Cancer Spectr. 2018;2(3):pky021. doi:10.1093/jncics/pky021
6. Research C for DE and. Hematology/Oncology (Cancer) Approvals & Safety Notifications. FDA. December 2019. https://www.fda.gov/drugs/resources-information-approved-drugs/hematologyoncology-cancer-approvals-safety-notifications. Accessed December 26, 2019.
7. Ferrari SM, Fallahi P, Galetta F, Citi E, Benvenga S, Antonelli A. Thyroid disorders induced by checkpoint inhibitors. Rev Endocr Metab Disord. 2018;19:325-333. doi:10.1007/s11154-018-9463-2
8. U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICE. Common Terminology Criteria for Adverse Events (CTCAE). Presented at the: November 27, 2017. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Accessed October 1, 2019.
9. González-Rodríguez E, Rodríguez-Abreu D, Spanish Group for Cancer Immuno-Biotherapy (GETICA). Immune Checkpoint Inhibitors: Review and Management of Endocrine Adverse Events. The Oncologist. 2016;21(7):804-816. doi:10.1634/theoncologist.2015-0509
10. Patel NS, Oury A, Daniels GA, Bazhenova L, Patel SP. Incidence of Thyroid Function Test Abnormalities in Patients Receiving Immune-Checkpoint Inhibitors for Cancer Treatment. The Oncologist. 2018;23(10):1236-1241. doi:10.1634/theoncologist.2017-0375
11. Jaafar J, Fernandez E, Alwan H, Philippe J. Programmed cell death-1 and programmed cell death ligand-1 antibodies-induced dysthyroidism. Endocr Connect. 2018;7(5):R196-R211. doi:10.1530/EC-18-0079
12. Di Giacomo AM, Danielli R, Calabrò L, et al. Ipilimumab experience in heavily pretreated patients with melanoma in an expanded access program at the University Hospital of Siena (Italy). Cancer Immunol Immunother CII. 2011;60(4):467-477. doi:10.1007/s00262-010-0958-2
13. Chang L-S, Barroso-Sousa R, Tolaney SM, Hodi FS, Kaiser UB, Min L. Endocrine Toxicity of Cancer Immunotherapy Targeting Immune Checkpoints. Endocr Rev. 2019;40(1):17-65.
14. Delivanis DA, Gustafson MP, Bornschlegl S, et al. Pembrolizumab-Induced Thyroiditis: Comprehensive Clinical Review and Insights Into Underlying Involved Mechanisms. J Clin Endocrinol Metab. 2017;102(8):2770-2780. doi:10.1210/jc.2017-00448
15. Osorio JC, Ni A, Chaft JE, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol Off J Eur Soc Med Oncol. 2017;28(3):583-589. doi:10.1093/annonc/mdw640
16. Azmat U, Liebner D, Joehlin-Price A, Agrawal A, Nabhan F. Treatment of Ipilimumab Induced Graves’ Disease in a Patient with Metastatic Melanoma. Case Rep Endocrinol. 2016;2016:2087525. doi:10.1155/2016/2087525
17. Mazarico I, Capel I, Giménez-Palop O, et al. Low frequency of positive antithyroid antibodies is observed in patients with thyroid dysfunction related to immune check point inhibitors. J Endocrinol Invest. 2019;42(12):1443-1450. doi:10.1007/s40618-019-01058-x
18. Lee H, Hodi FS, Giobbie-Hurder A, et al. Characterization of Thyroid Disorders in Patients Receiving Immune Checkpoint Inhibition Therapy. Cancer Immunol Res. 2017;5(12):1133-1140. doi:10.1158/2326-6066.CIR-17-0208
19. Yonezaki K, Kobayashi T, Imachi H, et al. Combination therapy of ipilimumab and nivolumab induced thyroid storm in a patient with Hashimoto’s disease and diabetes mellitus: a case report. J Med Case Reports. 2018;12. doi:10.1186/s13256-018-1708-x
20. Khan U, Rizvi H, Sano D, Chiu J, Hadid T. Nivolumab induced myxedema crisis. J Immunother Cancer. 2017;5:13. doi:10.1186/s40425-017-0213-x
21. Iyer PC, Cabanillas ME, Waguespack SG, et al. Immune-Related Thyroiditis with Immune Checkpoint Inhibitors. Thyroid Off J Am Thyroid Assoc. 2018;28(10):1243-1251. doi:10.1089/thy.2018.0116
22. Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497-1508. doi:10.1016/S1470-2045(16)30498-3
23. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23-34. doi:10.1056/NEJMoa1504030
24. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36(17):1714-1768. doi:10.1200/JCO.2017.77.6385
25. Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4(2):173-182. doi:10.1001/jamaoncol.2017.3064
26. Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014;6(230):230ra45. doi:10.1126/scitranslmed.3008002
27. Caturegli P, Di Dalmazi G, Lombardi M, et al. Hypophysitis Secondary to Cytotoxic T-Lymphocyte-Associated Protein 4 Blockade: Insights into Pathogenesis from an Autopsy Series. Am J Pathol. 2016;186(12):3225-3235. doi:10.1016/j.ajpath.2016.08.020
28. Situ Y, Chung L, Lee CS, Ho V. MRN (MRE11-RAD50-NBS1) Complex in Human Cancer and Prognostic Implications in Colorectal Cancer. Int J Mol Sci. 2019;20(4). doi:10.3390/ijms20040816
29. Piranavan P, Li Y, Brown E, Kemp EH, Trivedi N. Immune Checkpoint Inhibitor-Induced Hypoparathyroidism Associated With Calcium-Sensing Receptor-Activating Autoantibodies. J Clin Endocrinol Metab. 2019;104(2):550-556. doi:10.1210/jc.2018-01151
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2020 The Journal of Chulabhorn Royal Academy

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright and Disclaimer
Articles published in this journal are the copyright of Chulabhorn Royal Academy.
The opinions expressed in each article are those of the individual authors and do not necessarily reflect the views of Chulabhorn Royal Academy or any other faculty members of the Academy. The authors are fully responsible for all content in their respective articles. In the event of any errors or inaccuracies, the responsibility lies solely with the individual authors.


