Minimally Invasive Glaucoma Surgery (MIGS): A Glaucoma Procedure Revolution
Keywords:
MIGS, Glaucoma, DevicesAbstract
The principal treatment for eyes with glaucoma is reduction of intraocular pressure (IOP). Traditional glaucoma surgeries, such as trabeculectomy and glaucoma drainage device placement, are the most effective methods for achievement of target IOP after failed medication or laser therapy. However, these procedures are associated with possible sight-threatening adverse events, which involve cataract progression, suprachoroidal hemorrhage, hypotony maculopathy, and endophthalmitis. Minimally invasive glaucoma surgery (MIGS) utilizes innovative devices designed to reduce IOP, avoids medication side effects, and causes fewer complications than traditional surgeries. Some types of MIGS devices (e.g., Xen Gel Stents) have been used in many centers in Thailand. Because of the increasing options for treatment of patients with glaucoma, assessment of the advantages and disadvantages of MIGS devices may facilitate suitable treatment options for affected patients. Although MIGS remains a novel procedure, many studies have shown promising results regarding its ability to reduce IOP in a safe manner.
Downloads
References
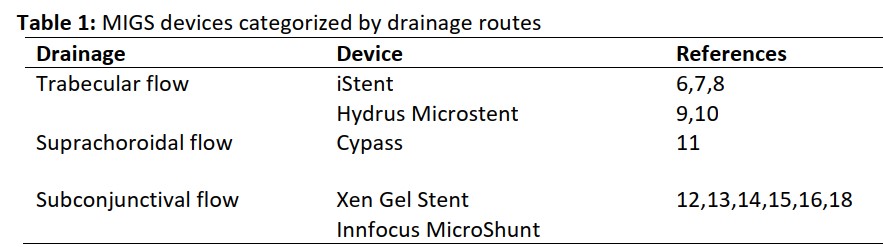
World Health Organization. WHO | Priority eye diseases. WHO. https://www.who.int/blindness/causes/priority/en/. Accessed February 15, 2020.
Gedde SJ, Schiffman JC, Feuer WJ, et al. The tube versus trabeculectomy study: design and baseline characteristics of study patients. Am J Ophthalmol. 2005;140(2):275-287. https://doi.org/10.1016/j.ajo.2005.03.031
Jampel HD, Musch DC, Gillespie BW, Lichter PR, Wright MM, Guire KE. Perioperative Complications of Trabeculectomy in the Collaborative Initial Glaucoma Treatment Study (CIGTS). Am J Ophthalmol. 2005;140(1):16-22. https://doi.org/10.1016/j.ajo.2005.02.013
American Academy of Ophthalmology. Basic and Clinical Science Course: Section 10: Glaucoma. Singapore: American Academy of Ophthalmology; 2008.
US Food and Drug Administration. Premarket Studies of Implantable Minimally Invasive Glaucoma Surgical (MIGS) Devices: Guidance for Industry and Food and Drug Administration Staff.; 2015. https://www.fda.gov/media/90950/download. Accessed July 1, 2018.
Samuelson TW, Katz LJ, Wells JM, Duh Y-J, Giamporcaro JE, US iStent Study Group. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118(3):459-467. https://doi.org/10.1016/j.ophtha.2010.07.007
Craven ER, Katz LJ, Wells JM, Giamporcaro JE, iStent Study Group. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012;38(8):1339-1345. https://doi.org/10.1016/j.jcrs.2012.03.025
Belovay GW, Naqi A, Chan BJ, Rateb M, Ahmed IIK. Using multiple trabecular micro-bypass stents in cataract patients to treat open-angle glaucoma. J Cataract Refract Surg. 2012;38(11):1911-1917. https://doi.org/10.1016/j.jcrs.2012.07.017
Samuelson TW, Chang DF, Marquis R, et al. A Schlemm Canal Microstent for Intraocular Pressure Reduction in Primary Open-Angle Glaucoma and Cataract: The HORIZON Study. Ophthalmology. 2019;126(1):29-37. https://doi.org/10.1016/j.ophtha.2018.05.012
Ahmed IIK, Fea A, Au L, et al. A Prospective Randomized Trial Comparing Hydrus and iStent Microinvasive Glaucoma Surgery Implants for Standalone Treatment of Open-Angle Glaucoma: The COMPARE Study. Ophthalmology. 2020;127(1):52-61. https://doi.org/10.1016/j.ophtha.2019.04.034
US Food and Drug Administration. Potential Eye Damage From Alcon CyPass Micro-Stent Used to Treat Open-Angle Glaucoma: FDA Safety Communication. FDA. September 2018. https://www.fda.gov/medical-devices/safety-communications/potential-eye-damage-alcon-cypass-micro-stent-used-treat-open-angle-glaucoma-fda-safety. Accessed September 14, 2018.
Sheybani A, Reitsamer H, Ahmed IIK. Fluid Dynamics of a Novel Micro-Fistula Implant for the Surgical Treatment of Glaucoma. Invest Ophthalmol Vis Sci. 2015;56(8):4789-4795. https://doi.org/10.1167/iovs.15-16625
Galal A, Bilgic A, Eltanamly R, Osman A. XEN Glaucoma Implant with Mitomycin C 1-Year Follow-Up: Result and Complications. J Ophthalmol. 2017;2017:5457246. https://doi.org/10.1155/2017/5457246
Downloads
Published
How to Cite
Issue
Section
License
Copyright and Disclaimer
Articles published in this journal are the copyright of Chulabhorn Royal Academy.
The opinions expressed in each article are those of the individual authors and do not necessarily reflect the views of Chulabhorn Royal Academy or any other faculty members of the Academy. The authors are fully responsible for all content in their respective articles. In the event of any errors or inaccuracies, the responsibility lies solely with the individual authors.


