Part 2: Occurrence of Racemic Natural Products and Their Biological Activities
Keywords:
Natural Products, Racemic mixture, Racemate, Biological activity, Marine natural productsAbstract
Part 2 review is a continuing version of Part 1, providing more examples of racemic natural products isolated from higher plants and microorganisms. The natural products reported here are from the research articles mostly published over the past few years, 2018-2020. This part also provides the information of marine racemic natural products, which had been isolated from marine invertebrates.
Downloads
References
Tang YQ, Li YQ, Xie YB, et al. Evodialones A and B: polyprenylated acylcyclopentanone racemates with a 3-ethyl-1,1-diisopentyl-4-methylcyclopentane skeleton from Evodia lepta. J Nat Prod. 2018;81(6):1483-1487.
Lee TH, Khan Z, Kim SY, Lee KR. Thiohydantoin and hydantoin derivatives from the roots of Armoracia rusticana and their neurotrophic and anti-neuroinflammatory activities. J Nat Prod. 2019;82(11):3020-3024.
Seow SLS, Hong SL, Lee GS, Malek SNA, Sabaratnam V. 6-Shogaol, a neuroactive compound of ginger (jahe gajah) induced neuritogenic activity via NGF responsive pathways in PC-12 cells. BMC Complement Altern Med. 2017;17(1):334.
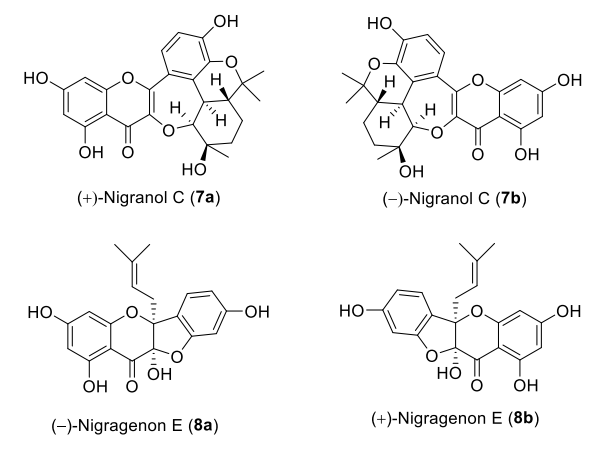
Xu L, Huang T, Huang C, Wu C, Jia A, Hu X. Chiral separation, absolute configuration, and bioactivity of two pairs of flavonoid enantiomers from Morus nigra. Phytochemistry. 2019;163:33-37.
Moelands SV, Lucassen PL, Akkermans RP, De Grauw WJ, Van de Laar FA. Alpha-glucosidase inhibitors for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst Rev. 2018;12:CD005061.
Van de Laar FA, Lucassen PL, Akkermans RP, Van de Lisdonk EH, Rutten GE, Van Weel C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005(2):CD003639.
Zolghadri S, Bahrami A, Hassan Khan MT, et al. A comprehensive review on tyrosinase inhibitors. J Enzyme Inhib Med Chem. 2019;34(1):279-309.
Xia Z, Xu TQ, Zhang HX, Chen YM, Xu W, Zhou GX. Bioactive sulfur-containing compounds from Xanthium sibiricum, including a revision of the structure of xanthiazinone. Phytochemistry. 2020;173:112293.
Lin B, Zhao Y, Han P, et al. Anti-arthritic activity of Xanthium strumarium L. extract on complete Freunds adjuvant induced arthritis in rats. J Ethnopharmacol. 2014;155(1):248-255.
Hsu F-L, Chen Y-C, Cheng J-T. Caffeic acid as active principle from the fruit of Xanthium strumarium to lower plasma glucose in diabetic rats. Planta Med. 2000;66(03):228-230.
Fan W, Fan L, Peng C, et al. Traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics and toxicology of Xanthium strumarium L.: A review. Molecules. 2019;24(2):359-399.
Ma Y-T, Huang M-C, Hsu F-L, Chang H-F. Thiazinedione from Xanthium strumarium. Phytochemistry. 1998;48(6):1083-1085.
Wang LY, Qiu BL, Xia H, et al. Yanhusanines A-F, isoquinoline-derived alkaloid enantiomers from Corydalis yanhusuo and their biological activity. J Nat Prod. 2020;83(2):489-496.
Yang D, Pearce RE, Wang X, Gaedigk R, Wan YJ, Yan B. Human carboxylesterases HCE1 and HCE2: ontogenic expression, inter-individual variability and differential hydrolysis of oseltamivir, aspirin, deltamethrin and permethrin. Biochem Pharmacol. 2009;77(2):238-247.
Adsersen A, Smitt UW, Simonsen HT, Christensen SB, Jaroszewski JW. Prenylated acetophenones from Melicope obscura and Melicope obtusifolia ssp. obtusifolia var. arborea and their distribution in Rutaceae. Biochem Syst Ecol. 2007;35(7):447-453.
Liu T, Liao H, Yuan K, Zhang Y. A new flavone from the Melicope patulinervia (Merr. & Chun) Huang. J Chem Res. 2012;36(1):31-33.
Vu VT, Chen XL, Kong LY, Luo JG. Melipatulinones A-C, three lignan-phloroglucinol hybrids from Melicope patulinervia. Org Lett. 2020;22(4):1380-1384.
Rajan L, Palaniswamy D, Mohankumar SK. Targeting obesity with plant-derived pancreatic lipase inhibitors: A comprehensive review. Pharmacol Res. 2020:104681.
Zang Y, Gong Y-H, Li X-W, et al. Canescones A–E: aromatic polyketide dimers with PTP1B inhibitory activity from Penicillium canescens. Org Chem Front. 2019;6(18):3274-3281.
Zinker BA, Rondinone CM, Trevillyan JM, et al. PTP1B antisense oligonucleotide lowers PTP1B protein, normalizes blood glucose, and improves insulin sensitivity in diabetic mice. Proc Natl Acad Sci USA. 2002;99(17):11357-11362.
Elchebly M, Payette P, Michaliszyn E, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283(5407):1544-1548.
Zhang S, Yang Q, Guo L, et al. Isolation, structure elucidation and racemization of (+)- and (-)-pratensilins A-C: unprecedented spiro indolinone-naphthofuran alkaloids from a marine Streptomyces sp. Chem Commun. 2017;53(72):10066-10069.
Chen Y, Zhang L, Zou G, et al. Anti-inflammatory activities of alkaloids from the mangrove endophytic fungus Phomopsis sp. SYSUQYP-23. Bioorg Chem. 2020;97:103712.
Liu H, Tan H, Wang W, et al. Cytorhizophins A and B, benzophenone-hemiterpene adducts from the endophytic fungus Cytospora rhizophorae. Org Chem Front. 2019;6(5):591-596.
Jimenez C. Marine natural products in medicinal chemistry. ACS Med Chem Lett. 2018;9(10):959-961.
Khalifa SAM, Elias N, Farag MA, et al. Marine natural products: A source of novel anticancer drugs. Mar Drugs. 2019;17(9):491.
Alves C, Silva J, Pinteus S, et al. From marine origin to therapeutics: The antitumor potential of marine algae-derived compounds. Front Pharmacol. 2018;9:777.
Carroll AR, Copp BR, Davis RA, Keyzers RA, Prinsep MR. Marine natural products. Nat Prod Rep. 2019;36(1):122-173.
Blunt JW, Carroll AR, Copp BR, Davis RA, Keyzers RA, Prinsep MR. Marine natural products. Nat Prod Rep. 2018;35(1):8-53.
Blunt JW, Copp BR, Keyzers RA, Munro MHG, Prinsep MR. Marine natural products. Nat Prod Rep. 2017;34(3):235-294.
Jiao W-H, Hong L-L, Sun J-B, et al. (±)-Hippolide J-A pair of unusual antifungal enantiomeric sesterterpenoids from the marine sponge Hippospongia lachne. Eur J Org Chem. 2017;2017(24):3421-3426.
Sun D-Y, Han G-Y, Yang N-N, Lan L-F, Li X-W, Guo Y-W. Racemic trinorsesquiterpenoids from the Beihai sponge Spongia officinalis: structure and biomimetic total synthesis. Org Chem Front. 2018;5(6):1022-1027.
Kiratisin P, Tucker KD, Passador L. LasR, a transcriptional activator of Pseudomonas aeruginosa virulence genes, functions as a multimer. J Bacteriol. 2002;184(17):4912-4919.
Zhu Y, Wang Y, Gu B-B, et al. Antifungal bromopyrrole alkaloids from the South China Sea sponge Agelas sp. Tetrahedron. 2016;72(22):2964-2971.
Mancini I, Guella G, Amade P, Roussakis C, Pietra F. Hanishin, a semiracemic, bioactive C9 alkaloid of the Axinellid Sponge Acanthella carteri from the Hanish Islands. A shunt metabolite? Tetrahedron Lett. 1997;38(35):6271-6274.
Srinivasa Reddy N, Venkateswarlu Y. S-(+)-Methyl ester of hanishin from the marine sponge Agelas ceylonica. Biochem Syst Ecol. 2000;28(10):1035-1037.
Downloads
Published
How to Cite
Issue
Section
License
Copyright and Disclaimer
Articles published in this journal are the copyright of Chulabhorn Royal Academy.
The opinions expressed in each article are those of the individual authors and do not necessarily reflect the views of Chulabhorn Royal Academy or any other faculty members of the Academy. The authors are fully responsible for all content in their respective articles. In the event of any errors or inaccuracies, the responsibility lies solely with the individual authors.


