Biological Activities of Extract from Watermelon rind Citrullus Lanatus (Thunb.) Matsum and Nakai
Keywords:
Biological Activities, Watermelon rindAbstract
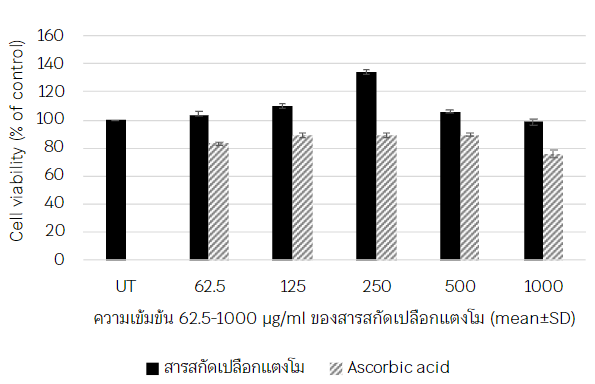
Background: Watermelon rind is a waste that is not utilized, contains important groups of vitamin C, fiber, and citrulline, which is an antioxidant and has a significant benefit to the human body. Objectives: The aim of this study was to investigate the biological activities of the 85% ethanolic extracts of watermelon rind (Citrullus Lanatus (Thunb.) Matsum and Nakai) Methods: These study were in vitro test. The total phenolic content and antioxidant activity were evaluated according to DPPH, ABTS and FRAP assays. Human Forehead Fibroblast Cells (HFF) of cell proliferation by MTT assay and collagen content analysis by Hydroxyproline Assay from cell culture media with extracts. Results: The results showed that 85% ethanolic extracts of watermelon rind (Citrullus Lanatus (Thunb.) Matsum and Nakai) had total phenolic content was 32.60 ± 0.84 µg GAE/mg extracts, antioxidant activities by DPPH and ABTS calculated in IC 50 833.40 ± 2.46, 1655.48 ± 0.05 µg/ml, respectively. The FRAP value was 0.23 ± 0.03 mM Fe (II)/mg. The HFF cell viability increased with increasing concentration of watermelon rind extract at 62.5-500 µg/ml but cell viability will be decreased at than 500 µg / ml. The highest collagen content was 256.79±24.69 µg/ml and concentration at 1000 µg/ml showed the highest collagen Conclusions: The extracts of watermelon rind (Citrullus Lanatus (Thunb.) Matsum and Nakai) had total phenolic compounds, antioxidant activity and collagen stimulating activity in HFF cell culture, therefore these extracts the develop for cosmeceutical products in the future.
Downloads
References
Sadef Y, Javed T, Javed R, et al. Nutritional status, antioxidant activity and total phenolic content of different fruits and vegetables’ peels. PLoS One. 2022;17 (5):e0265566. Published 2022 May 12. doi:10.1371/journal.pone.0265566
Ho LH, Ramli N, Tan TC, Muhamad N, Haron MN. Effect of Extraction Solvents and Drying Conditions on Total Phenolic Content and Antioxidant Properties of Watermelon Rind Powder.Sains Malaysiana. 2018;47:99-107. doi:10.17576/jsm-2018-4701-12
Olayinka U, Etejere EO. Proximate and chemical compositions of watermelon (Citrullus lanatus (Thunb.) Matsum and Nakai cv red and cucumber (Cucumis sativus L. cv Pipino). International Food Research Journal. 2018;25:1060-1066.
Manivannan A, Lee ES, Han K, Lee HE, Kim DS. Versatile Nutraceutical Potentials of Watermelon—A Modest Fruit Loaded with Pharmaceutically Valuable Phytochemicals. Molecules. 2020;25: 5258. doi:10.3390/molecules25225258
Gu I, Balogun O, Brownmiller C, Kang HW, Lee S-O. Bioavailability of Citrulline in Watermelon Flesh, Rind, and Skin Using a Human Intestinal Epithelial Caco-2 Cell Model. Applied Sciences. 2023; 13(8):4882. https://doi.org/10.3390/app13084882
Purwidyaningrum I, Iswandi, Billi J. Diuretic activity of water melon rind extract (citrullus vulgaris) and its influence on sodium and potassium levels.Int. J. Pharmacogn. Phytochem. Res. 2018;10(5):213-215.doi:10.25258/phyto. 10.5.6
Maoto M, Beswa D, Jideani A. Watermelon as a potential fruit snack. International Journal of Food Properties. 2019;22: 355-370. doi:10.1080/10942912.2019.1584212
Aderiye BI, David OM, Fagbohun ED, et al. Immunomodulatory and phytomedicinal properties of watermelon juice and pulp (Citrullus lanatus Linn): A review. GSC biol. pharm. sci. 2020;11(02):153– 165. doi:10.30574/gscbps.2020.11.2.0079
Ministry of Agriculture and Cooperatives. Thai agricultural standard TAS 20-2012 watermelon. Published 2012 Nov 16.
Ahn J, Choi W, Kim S, Ha TY. Anti-diabetic effect of watermelon (Citrullus vulgaris Schrad) on Streptozotocin-induced diabetic mice. Food science and biotechnology. 2011;20:251-254. doi:10.1007/s10068-011-0034-5
Moeiklang N, Ruangviriyachai C, Determination of phenolic compounds and antioxidant potential in fruit beverages. KKU Res J (GS). 2014;14(4):69-79.
Musa KH, Abdullah A, Kuswandi B, Hidayat MA. A novel high throughput method based on the DPPH dry reagent array for determination of antioxidant activity. Food Chem. 2013;141(4):4102-4106. doi:10.1016/j.foodchem.2013.06.112
Berker KI, Güçlü K, Tor I, Apak R. Comparative evaluation of Fe(III) reducing power-based antioxidant capacity assays in the presence of phenanthroline, bathophenanthroline, tripyridyltriazine (FRAP), and ferricyanide reagents. Talanta. 2007;72(3):1157-1165. doi:10.1016/j.talanta.2007.01.019
Duan XJ, Zhang WW, Li XM, et al. Evaluation of antioxidant property of extract and fractions obtained from a red alga, polysiphonia urceolata. Food Chem. 2005;95(1):37-43.
สถาพร สัตย์ซื่ อ, สุธาสินี ทัพพสารพงศ์, ธีระศักดิ์ ดำารงรุ่งเร ือง.ฤทธิ์ต้านออกซิเดชัน และฤทธิกระตุ้นการสร้างคอลลาเจนในเซลล์ ์ ไฟโบรบลาสต์จากช่องปากของสารสกัดจากพืช. วารสารเภสัชศาสตร์อีสาน. 2557;9:38-43.
Kim CH, Park MK, Kim SK, et al. Antioxidant capacity and anti-inflammatory activity of lycopene in watermelon. Int J Food Sci Technol. 2014;49:2083–2091.
Wanichpakorn S, Kedjarune-Laggat U. Primary cell culture from human oral tissue: Gingival keratinocytes, gingival fibroblasts and periodontal ligament fibroblasts. Songklanakarin Journal of Science and Technology. 2010;32:327-331.
Łopusiewicz Ł, Macieja S, Sliwi ´nski M, et al. Alginate biofunctional films modified with melanin from watermelon seeds and zinc oxide/silver nanoparticles. Materials. 2022;15(2381):1-23.doi:10.3390/ma15072381
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Chulabhorn Royal Academy

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright and Disclaimer
Articles published in this journal are the copyright of Chulabhorn Royal Academy.
The opinions expressed in each article are those of the individual authors and do not necessarily reflect the views of Chulabhorn Royal Academy or any other faculty members of the Academy. The authors are fully responsible for all content in their respective articles. In the event of any errors or inaccuracies, the responsibility lies solely with the individual authors.


