BCOR-CCNB3 fusion-positive soft tissue sarcoma: A case report and literature review
Keywords:
BCOR-CCNB3 sarcoma, undifferentiated sarcoma, acute myeloblastic leukemiaAbstract
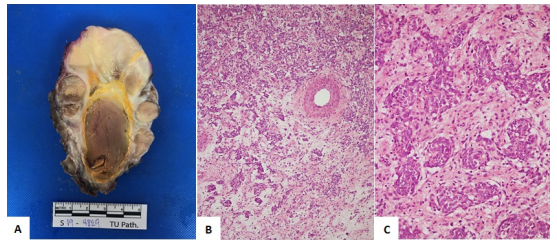
BCOR-CCNB3 sarcoma is one of a group of undifferentiated sarcomas. The objective of this case report was to describe the clinical and pathological data of a patient with this tumor. Our patient was a 14-year-old male adolescent with right flank pain. Imaging revealed a psoas muscle mass lesion. Histology and gene expression were suggestive of BCOR-CCNB3 sarcoma. Chemotherapy, surgery, and radiotherapy were used according to the Ewing sarcoma protocol in Thailand. Unfortunately, our patient died from acute myeloblastic leukemia (M5) without remission despite chemotherapy.
Downloads
References
Pierron G, Tirode F, Lucchesi C, et al. A new subtype of bone sarcoma defined by BCOR-CCNB3 gene fusion. Nat Genet. 2012;44(4):461-466. https://doi.org/10.1038/ng.1107
Cohen-Gogo S, Cellier C, Coindre JM, et al. Ewing-like sarcomas with BCOR-CCNB3 fusion transcript: a clinical, radiological and pathological retrospective study from the Société Française des Cancers de L'Enfant. Pediatr Blood Cancer. 2014;61(12):2191-2198. https://doi.org/10.1002/pbc. 25210
Puls F, Niblett A, Marland G, et al. BCOR-CCNB3 (Ewing-like) sarcoma: a clinicopathologic analysis of 10 cases, in comparison with conventional Ewing sarcoma. Am J Surg Pathol. 2014;38(10):1307-1318. https://doi.org /10.1097/PAS. 0000000000000223
Peters TL, Kumar V, Polikepahad S, et al. BCOR-CCNB3 fusions are frequent in undifferentiated sarcomas of male children. Mod Pathol. 2015;28(4):575-586. https://doi.org/10.1038/modpathol.2014.139
Shibayama T, Okamoto T, Nakashima Y, et al. Screening of BCOR-CCNB3 sarcoma using immunohistochemistry for CCNB3: A clinicopathological report of three pediatric cases. Pathol Int. 2015;65(8):410-414. https://doi.org /10.1111/ pin.12319
Li WS, Liao IC, Wen MC, Lan HH, Yu SC, Huang HY. BCOR-CCNB3-positive soft tissue sarcoma with round-cell and spindle-cell histology: a series of four cases highlighting the pitfall of mimicking poorly differentiated synovial sarcoma. Histopathology. 2016;69(5):792-801. https://doi.org/10.1111/his.13001
Ludwig K, Alaggio R, Zin A, et al. BCOR-CCNB3 undifferentiated sarcoma-does immunohistochemistry help in the identification? Pediatr Dev Pathol. 2017;20(4):321-329. https://doi.org/10.1177/1093526617698263
Matsuyama A, Shiba E, Umekita Y, et al. Clinicopathologic diversity of undifferentiated sarcoma with BCOR-CCNB3 fusion: analysis of 11 cases with a reappraisal of the utility of immunohistochemistry for BCOR and CCNB3. Am J Surg Pathol. 2017;41(12):1713-1721. https://doi: 10.1097/PAS.0000 000000000934
Kao YC, Owosho AA, Sung YS, et al. BCOR-CCNB3 fusion positive sarcomas: a clinicopathologic and molecular analysis of 36 cases with comparison to morphologic spectrum and clinical behavior of other round cell sarcomas. Am J Surg Pathol. 2018;42(5):604-615. https://doi.org/10.1097/PAS.00000000 00000965
The Thai Society of Hematology. National protocol for the treatment of childhood cancers 2016. Bangkok: Sahamitr Printing and Publishing; 2016.
Alfaro-Cervello C, Andrade-Gamarra V, Nieto G, et al. Congenital undifferentiated sarcoma associated to BCOR-CCNB3 gene fusion. Pathol Res Pract. 2017;213(11):1435-1439. https://doi.org/10.1016/j.prp.2017.07.012
Argani P, Kao YC, Zhang L, et al. Primary renal sarcomas with BCOR-CCNB3 gene fusion: a report of 2 cases showing histologic overlap with clear cell sarcoma of kidney, suggesting further link between BCOR-related sarcomas of the kidney and soft tissues. Am J Surg Pathol. 2017;41(12):1702-1712. https://doi.org/10.1097/PAS.0000000000000926
Wong MK, Ng CCY, Kuick CH, et al. Clear cell sarcomas of the kidney are characterised by BCOR gene abnormalities, including exon 15 internal tandem duplications and BCOR-CCNB3 gene fusion. Histopathology. 2018;72(2):320-329. https://doi.org/10.1111/his.13366
Felix CA. Leukemias related to treatment with DNA topoisomerase II inhibitors. Med Pediatr Oncol. 2001;36(5):525-535. https://doi.org/10.1002 /mpo.1125
Davies SM. Therapy-related leukemia associated with alkylating agents. Med Pediatr Oncol. 2001;36(5):536-540. https://doi.org/10.1002/mpo.1126
Hijiya N, Ness KK, Ribeiro RC, Hudson MM. Acute leukemia as a secondary malignancy in children and adolescents: current findings and issues. Cancer. 2009;115(1):23-35. https://doi.org/10.1002/cncr.23988
Hijiya N, Hudson MM, Lensing S, et al. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA. 2007;297(11):1207-1215. https://doi.org/10.1001/jama.297.11.1207
Pui CH, Ribeiro RC, Hancock ML, et al. Acute myeloid leukemia in children treated with epipodophyllotoxins for acute lymphoblastic leukemia. N Engl J Med. 1991;325(24):1682-1687. https://doi.org/10.1056/NEJM19911212325240 2
Hijiya N, Gajjar A, Zhang Z, et al. Low-dose oral etoposide-based induction regimen for children with acute lymphoblastic leukemia in first bone marrow relapse. Leukemia. 2004;18(10):1581-1586. https://doi.org/10.1038/sj. leu.2403 467
Sandoval C, Pui CH, Bowman LC, et al. Secondary acute myeloid leukemia in children previously treated with alkylating agents, intercalating topoisomerase II inhibitors, and irradiation. J Clin Oncol. 1993;11(6):1039-1045. https://doi.org/10.1200/JCO.1993.11.6.1039
Pedersen-Bjergaard J, Philip P, Larsen SO, et al. Therapy-related myelodysplasia and acute myeloid leukemia. Cytogenetic characteristics of 115 consecutive cases and risk in seven cohorts of patients treated intensively for malignant diseases in the Copenhagen series. Leukemia. 1993;7(12):1975-1986.
Kushner BH, Cheung NK, Kramer K, Heller G, Jhanwar SC. Neuroblastoma and treatment-related myelodysplasia/leukemia: the Memorial Sloan-Kettering experience and a literature review. J Clin Oncol. 1998;16(12):3880-3889. https://doi.org/10.1200/JCO.1998.16.12.3880
Bhatia S, Krailo MD, Chen Z, et al. Therapy-related myelodysplasia and acute myeloid leukemia after Ewing sarcoma and primitive neuroectodermal tumor of bone: A report from the Children's Oncology Group. Blood. 2007;109(1):46-51. https://doi.org/10.1182/blood-2006-01-023101
Smith MA, Rubinstein L, Anderson JR, et al. Secondary leukemia or myelodysplastic syndrome after treatment with epipodophyllotoxins. J Clin Oncol. 1999;17(2):569-577. https://doi.org/10.1200/JCO.1999.17.2.569
Kyriazoglou A, Bagos P. Meta-analysis of BCOR rearranged sarcomas: challenging the therapeutic approach. Acta Oncol. 2021;60(6):721-726. https://doi.org/10.1080/0284186X.2021.1890818
Grossmann V, Tiacci E, Holmes AB, et al. Whole-exome sequencing identifies somatic mutations of BCOR in acute myeloid leukemia with normal karyotype. Blood. 2011;118(23):6153-63. https://doi: 10.1182/blood-2011-07-365320
Yamato A, Soda M, Ueno T, et al. Oncogenic activity of BIRC2 and BIRC3 mutants independent of nuclear factor-κB-activating potential. Cancer Sci. 2015;106(9):1137-1142. https://doi.org/10.1111/cas.12726
Hijiya N, Ness KK, Ribeiro RC, et al. Acute leukemia as a secondary malignancy in children and adolescents: current findings and issues. Cancer. 2009;115(1):23–35. https://doi:10.1002/cncr.23988
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Chulabhorn Royal Academy

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright and Disclaimer
Articles published in this journal are the copyright of Chulabhorn Royal Academy.
The opinions expressed in each article are those of the individual authors and do not necessarily reflect the views of Chulabhorn Royal Academy or any other faculty members of the Academy. The authors are fully responsible for all content in their respective articles. In the event of any errors or inaccuracies, the responsibility lies solely with the individual authors.


