Nursing care for people with Neuroendocrine Tumor (NETs) receiving 177Lu-DOTATATE Therapy
Keywords:
177Lu-DOTATATE NETs Nursing careAbstract
Neuroendocrine tumors (NETs) are cancers of the endocrine cells, originating in organs such as lungs, stomach, small intestine, colon, or pancreas and spreading to other organs. Whilst, treatment modalities of NETs comprise surgery, chemotherapy and novel innovative drugs to targeted organs, including the use of radiopharmaceutical agents. 177Lu-DOTATATE is an alternative and efficacious treatment with increasing survival rates and few side effects. Nursing care of NETs patients with 177Lu-DOTATATE therapy based on a specialized knowledge on disease pathology and radiation in order to increase patient safety and enhance efficiency effectiveness of 177Lu-DOTATATE therapy.
Downloads
References
Zhang J, Song Q, Cai L, Xie Y, Chen Y. The efficacy of 177Lu-DOTATATE peptide receptor radionuclide therapy (PRRT) in patients with metastatic neuroendocrine tumours: a systematic review and meta-analysis. J Cancer Res Clin Oncol .2020;146(6):1533-1543.
หน่วยสารสนเทศมะเร็งโรงพยาบาลสงขลานครินทร์. มะเร็งเน็ท ก้อนเนื้อร้าย อาการคล้ายโรคอื่น. 2556. วันที่ 10 เมษายน 2566. จาก http://medinfo2.psu.ac.th/cancer/db/news_showpic.php ?newsID=853&tyep_ID=2.
Abbott A, Sakellis CG, Andersen E, Kuzuhara Y, Gilbert L, Boyle K, et al. Guidance on 177Lu-DOTATATE Peptide Receptor Radionuclide Therapy from the Experience of a Single Nuclear Medicine Division. J Nucl Med Technol. 2018;46(3):237-244.
National Cancer Institute. FAD Approves new treatment for certain Neuroendocrine tumors.2018. Accessed April 10, 2022. https://www.cancer.gov/news-events/cancer-currents-blog/2018/lutathera-fda-gastrointestinal-nets.
Hennrich U, Kopka K. Lutathera®: The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharmaceuticals. 2019;12(3):114.
Demirci E, Kabasakal L, Toklu T, Ocak M, Şahin OE, Alan-Selcuk N, et al. 177Lu-DOTATATE therapy in patients with neuroendocrine tumours including high-grade (WHO G3) neuroendocrine tumours. Nucl Med Commun. 2018;39(8):789-796.
Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376(2):125-135
Kam BLR, Teunissen JJM, Krenning EP, de Herder WW, Khan S, van Vliet EI, et al. Lutetium-labelled peptides for therapy of neuroendocrine tumours. E Eur J Nucl Med Mol Imaging. 2012;39(S1):103-112
Schmitt A, Bernhardt P, Nilsson O, Ahlman H, Kolby L, Schmitt J, et al. Biodistribution and Dosimetry of 177Lu-Labeled [DOTA, Tyr3] Octeotate in Male Nude Mice with Human Small Cell Lung Cancer. Cancer Biother Radiopharm. 2003;18(4):593-599.
Zaknun JJ, Bodei L, Mueller-Brand J, Pavel ME, Baum RP, Hörsch D, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40(5):800-816.
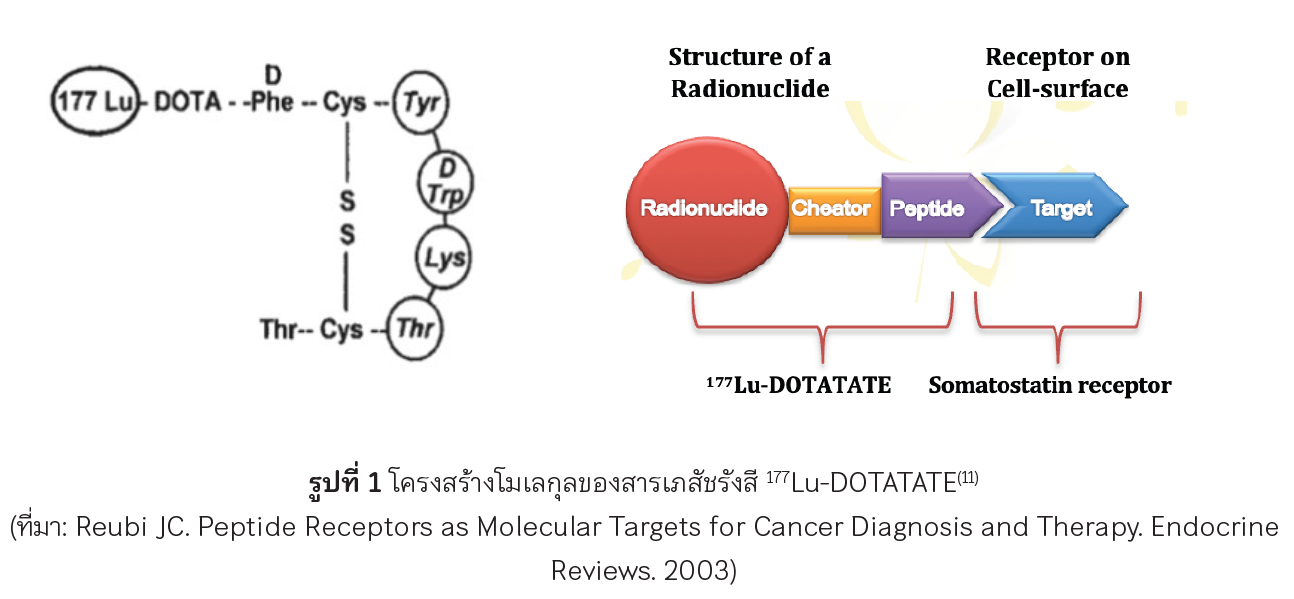
Reubi JC. Peptide Receptors as Molecular Targets for Cancer Diagnosis and Therapy. Endocr Rev. 2003;24(4):389-427.
Medscape. Lutetium: Lu-177 DOTATATE (Rx). Accessed April 22, 2022. https://reference. medscape.com/drug/lutathera-lutetium-lu-177-dota-tate-1000113.
Kendi AT, Halfdanarson TR, Packard A, Dundar A, Subramaniam RM. Therapy with 177Lu-DOTATATE: Clinical Implementation and Impact on Care of Patients With Neuroendocrine Tumors. AJR Am J Roentgenol. 2019;213(2):309-317.
Hope TA, Abbott A, Colucci K, Bushnell DL, Gardner L, Graham WS, et al. NANETS/SNMMI Procedure Standard for Somatostatin Receptor–Based Peptide Receptor Radionuclide Therapy with 177Lu-DOTATATE. J Nucl Med Technol 2019; 60(7):937-943.
Burkett BJ, Dundar A, Young JR, Packard AT, Johnson GB, Halfdanarson TR, et al. How We Do It: A Multidisciplinary Approach to 177Lu DOTATATE Peptide Receptor Radionuclide Therapy. Radiology. 2021;298(2):261-274.
The American College of Radiology, ACR-ACNM-ASTRO-SNMMI Practice parameter for Lutetium-177 (Lu-177)DOTATATE therapy. 2020. Accessed April 28, 2022. https://www.acr.org/-/media/ACR/Files/Practice-Parameters/ Lutetium-177-DOTATATE. pdf.
Hennrich, U., & Kopka, K. Lutathera®: the first FDA-and EMA-approved radiopharmaceutical for peptide receptor radionuclide therapy. Pharmaceuticals, (2019);12 (3):114.
Lisa A. Thompson, PharmD, BCOP, Oncology Nurse Advisor: Significance of Amino Acid Solution with Lutetium Lu 177 Dotatate. Accessed Februry 7, 2019 https://www.oncologynurseadvisor.com/home/departments/advisor-forum/signify cance-of-amino-acid-solution-with-lutetium-lu-177-dotatate.
Brabander T, van der Zwan WA, etal. Long-Term Efficacy, Survival, and Safety of [177Lu-DOTAO,Tyr3] octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin Cancer Res. 2017 Aug 15;23(16):4617-462
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Chulabhorn Royal Academy

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright and Disclaimer
Articles published in this journal are the copyright of Chulabhorn Royal Academy.
The opinions expressed in each article are those of the individual authors and do not necessarily reflect the views of Chulabhorn Royal Academy or any other faculty members of the Academy. The authors are fully responsible for all content in their respective articles. In the event of any errors or inaccuracies, the responsibility lies solely with the individual authors.


