A survey of post-transplant direct non-medical and indirect costs for recipients and caregivers at five transplant centers in Thailand
Keywords:
Direct non-medical costs, Indirect costs, Transplantation, Kidney, Bone marrowAbstract
Background: A previous study in Thailand examined the cost-effectiveness model of an oral form of anti-cytomegalovirus drug, valganciclovir, versus intravenous ganciclovir in post-transplant care for national policy decision. Due to valganciclovir gave lower costs for hospital visits, but higher drug costs than ganciclovir. This study therefore presented the direct non-medical and indirect costs from the same study, to enable a fuller description how these cost parameters simulated in the model came from.
Methods: A total of 87 kidney and 67 bone marrow transplant recipients in Thailand were followed-up 1 year after transplantation at three kidney and two bone marrow transplant centers. They were surveyed to identify the direct non-medical costs arising from their transplant. These included out-of-pocket payments for traveling to centers, food during visits, and hotel stays. Patient and caregiver productivity losses were included as indirect costs using the human capital approach and estimated from Thai Gross National Income per capita. Mean and standard error (SE) were used to estimate all costs.
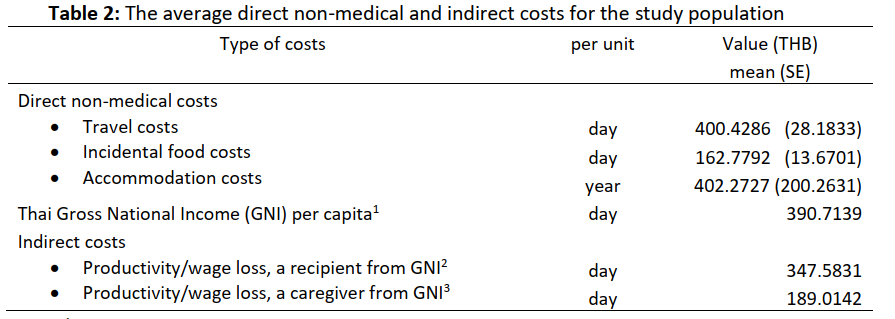
Results: The mean daily traveling costs were 400.4286 Thai baht (THB) (SE = 28.1833). The incidental daily costs for food were 162.7792 THB (SE = 13.6701). The annual accommodation cost was 402.2727 THB (SE = 200.2631), and the individual daily productivity loss was estimated as 390.7139 THB for patients and 189.0142 THB for caregivers.
Conclusions: This study identified the unit costs for patients visiting hospitals during 1 year of post-transplant care. These costs can be used to supplement information about the management patterns for valganciclovir or ganciclovir modelling, and may also be useful in economic evaluation of other post-transplant care for future decision-making in Thailand.
Downloads
References
Social Security Office. Social Security Benefit Scheme. Benefit package of renal replacement therapy. Ministry of Labour. Published 2017. https://www.sso.go.th/wpr/main/service/กองทุนประกันสังคม_detail_detail_1_125_0/23. In Thai.
Social Security Office, Comptroller General’s Department, National Health Security Office (NHSO). Manual of three scheme integration, universal coverage-social security benefit-civil servant medical benefit scheme. National Health Security Office (NHSO); 2018. https://www.nhso.go.th/files/userfiles/file/2018/001/คู่มือ%20ถาม-ตอบ%20บูรณาการ%203%20กองทุน%20ทั้งเล่ม%20(เผยแพร่).pdf. In Thai.
Ghanta M, Jim B. Renal Transplantation in Advanced Chronic Kidney Disease Patients. Med Clin North Am. 2016;100(3):465-476. https://doi.org/10.1016/j.mcna.2015.12.003
Thai Transplantation Society. Annual Report 2016 Kidney Transplantation in Thailand.; 2016. http://www.transplantthai.org/upload/170905105531507_MNB.pdf. In Thai.
Social Security Office. Social security benefit scheme for bone marrow transplantation. Ministry of Labour. Published 2017. https://www.sso.go.th/wpr/main/service/กองทุนประกันสังคม_detail_detail_1_125_0/19. In Thai.
Eisenberg JM. Clinical economics. A guide to the economic analysis of clinical practices. JAMA. 1989;262(20):2879-2886. https://doi.org/10.1001/jama.262.20.2879
Weeda ER, Su Z, Taber DJ, et al. Hospital admissions and emergency department visits among kidney transplant recipients. Clin Transplant. 2019;33(5):e13522. https://doi.org/10.1111/ctr.13522
Kim M, Martin ST, Townsend KR, Gabardi S. Antibody-mediated rejection in kidney transplantation: a review of pathophysiology, diagnosis, and treatment options. Pharmacotherapy. 2014;34(7):733-744. https://doi.org/10.1002/phar.1426
Gabardi S, Asipenko N, Fleming J, et al. Evaluation of Low- Versus High-dose Valganciclovir for Prevention of Cytomegalovirus Disease in High-risk Renal Transplant Recipients. Transplantation. 2015;99(7):1499-1505. https://doi.org/10.1097/TP.0000000000000570
Dounousi E, Leivaditis K, Eleftheriadis T, Liakopoulos V. Osteoporosis after renal transplantation. Int Urol Nephrol. 2015;47(3):503-511. https://doi.org/10.1007/s11255-014-0862-3
Tanriover B, Wright SE, Foster SV, et al. High-dose intravenous immunoglobulin and rituximab treatment for antibody-mediated rejection after kidney transplantation: a cost analysis. Transplant Proc. 2008;40(10):3393-3396. https://doi.org/10.1016/j.transproceed.2008.08.131
Momin F, Chandrasekar PH. Antimicrobial prophylaxis in bone marrow transplantation. Ann Intern Med. 1995;123(3):205-215. https://doi.org/10.7326/0003-4819-123-3-199508010-00008
Goa KL, Bryson HM. Recombinant granulocyte-macrophage colony-stimulating factor (rGM-CSF): an appraisal of its pharmacoeconomic status in neutropenia associated with chemotherapy and autologous bone marrow transplant. PharmacoEconomics. 1994;5(1):56-77. https://doi.org/10.2165/00019053-199405010-00008
Stewart A, Powles R, Hewetson M, Antrum J, Richardson C, Mehta J. Costs of antifungal prophylaxis after bone marrow transplantation. A model comparing oral fluconazole, liposomal amphotericin and oral polyenes as prophylaxis against oropharyngeal infections. PharmacoEconomics. 1995;8(4):350-361. https://doi.org/10.2165/00019053-199508040-00009
Sangmala P. The systematic review of economic evaluation of oral valganciclovir and intravenous ganciclovir for cytomegalovirus (CMV) prevention and treatment in transplant recipients. J Health Syst Res. 2018;12(3):500-514.
Luan FL, Kommareddi M, Ojo AO. Universal prophylaxis is cost effective in cytomegalovirus serology-positive kidney transplant patients. Transplantation. 2011;91(2):237-244. https://doi.org/10.1097/TP.0b013e318200000c
Luan FL, Stuckey LJ, Park JM, Kaul D, Cibrik D, Ojo A. Six-month prophylaxis is cost effective in transplant patients at high risk for cytomegalovirus infection. J Am Soc Nephrol JASN. 2009;20(11):2449-2458. https://doi.org/10.1681/ASN.2008111166
Wiltshire H, Hirankarn S, Farrell C, et al. Pharmacokinetic profile of ganciclovir after its oral administration and from its prodrug, valganciclovir, in solid organ transplant recipients. Clin Pharmacokinet. 2005;44(5):495-507. https://doi.org/10.2165/00003088-200544050-00003
Martin DF, Sierra-Madero J, Walmsley S, et al. A controlled trial of valganciclovir as induction therapy for cytomegalovirus retinitis. N Engl J Med. 2002;346(15):1119-1126. https://doi.org/10.1056/NEJMoa011759
Issaragrisil S. Hematopoietic stem cell transplantation in Thailand. Bone Marrow Transplant. 2008;42 Suppl 1:S137-S138. https://doi.org/10.1038/bmt.2008.142
Yamane T. Statistics: An Introductory Analysis. 3d ed. Harper & Row; 1973.
Oppenheimer F, Gonzalez-Molina M, Rubio M. Cost of prophylaxis in the management of cytomegalovirus infection in solid organ transplant recipients. Clin Transplant. 2007;21(4):441-448. https://doi.org/10.1111/j.1399-0012.2007.00612.x
Mercado-Martínez FJ, Hernández-Ibarra E, Ascencio-Mera CD, Díaz-Medina BA, Padilla-Altamira C, Kierans C. [Kidney transplant patients without social protection in health: what do patients say about the economic hardships and impact?]. Cad Saude Publica. 2014;30(10):2092-2100. https://doi.org/10.1590/0102-311x00150713
Helanterä I, Isola T, Lehtonen TK, Åberg F, Lempinen M, Isoniemi H. Association of Clinical Factors with the Costs of Kidney Transplantation in the Current Era. Ann Transplant. 2019;24:393-400. https://doi.org/10.12659/AOT.915352
Chamberlain G, Baboolal K, Bennett H, et al. The economic burden of posttransplant events in renal transplant recipients in Europe. Transplantation. 2014;97(8):854-861. https://doi.org/10.1097/01.TP.0000438205.04348.69
Snowsill TM, Moore J, Mujica Mota RE, et al. Immunosuppressive agents in adult kidney transplantation in the National Health Service: a model-based economic evaluation. Nephrol Dial Transplant. 2017;32(7):1251-1259. https://doi.org/10.1093/ndt/gfx074
Cremaschi L, von Versen R, Benzing T, et al. Induction therapy with rabbit antithymocyte globulin versus basiliximab after kidney transplantation: a health economic analysis from a German perspective. Transpl Int Off J Eur Soc Organ Transplant. 2017;30(10):1011-1019. https://doi.org/10.1111/tri.12991
Downloads
Published
How to Cite
Issue
Section
License
Copyright and Disclaimer
Articles published in this journal are the copyright of Chulabhorn Royal Academy.
The opinions expressed in each article are those of the individual authors and do not necessarily reflect the views of Chulabhorn Royal Academy or any other faculty members of the Academy. The authors are fully responsible for all content in their respective articles. In the event of any errors or inaccuracies, the responsibility lies solely with the individual authors.


