Genomic characterization for secondary metabolite biosynthetic genes of Microbispora sp. KK1-11
Keywords:
Microbispora, Whole-genome sequence analysis, Actinomycete, Secondary metabolitesAbstract
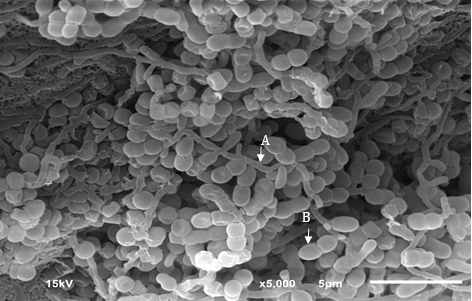
Background: Rare actinomycetes, especially genus Microbispora, have attracted attention because of their ability to produce various bioactive secondary metabolites. Many valuable secondary metabolites, such as bispolides, linfuranone A, and microbiaeratin, produced by Microbispora spp. have been reported, and there is great interest in efforts to discover new Microbispora spp. with the ability to produce such metabolites. The application of in silico biosynthetic predictions from genome mining data is usually used to identify promising Microbispora spp. in nature. In this study, we used existing genomic data to characterize the taxonomic position of Microbispora sp. KK1-11 and to identify biosynthetic gene clusters (BGCs) in the genome.
Methods: An actinomycete strain KK1-11 was taxonomically characterized using a polyphasic approach. To confirm the taxonomic classification of the strain at the genus level, morphological, chemotaxonomic, and 16S rRNA gene sequence analyses were performed. Whole-genome shotgun sequencing was carried out using an Illumina MiSeq 1TB platform. The genomic data were evaluated for the production of secondary metabolites using the antiSMASH platform and the antibacterial activity of metabolites was also assessed.
Results: Actinomycete strain KK1-11 was taxonomically characterized as a member of the genus Microbispora. Genomic-based identification revealed that while KK1-11 is most closely related to Microbispora catharanthi CR1-09T, the average nucleotide identity value was low at 94.44%. Genome analysis by antiSMASH revealed that KK1-11 contains several secondary metabolite BCGs (smBGCs), including type I and III polyketide synthase, terpene biosynthesis, non-ribosomal peptide synthetase (NRPS), and NRPS-like gene clusters. In addition, several smBGCs in the genome of KK1-11 showed no relatedness to known clusters.
Conclusion: This study demonstrates that Microbispora sp. KK1-11 may represent a novel member of the genus Microbispora capable of producing new secondary metabolites, representing a soil actinomycete with the potential to produce valuable bioactive compounds.
Downloads
References
Lewin GR, Carlos C, Chevrette MG, Horn HA, McDonald BR, Stankey RJ, Fox BG, Currie CR. Evolution and ecology of actinobacteria and their bioenergy applications. Annu Rev Microbiol. 2016; 70:235–254.
Agrawal P K, Agrawal S, Shrivastava R. Modern molecular approaches for analyzing microbial diversity from mushroom compost ecosystem. 3 Biotech. 2015;5(6):853–866.
Yagi A, Uchida R, Hamamoto H, Sekimizu K, Kimura K-I, Tomoda H. Anti-Mycobacterium activity of microbial peptides in a silkworm infection model with Mycobacterium smegmatis. J antibiot. 2017;70(5):685–690.
Okujo N, Iinuma H, George A, Eim KR, Li TL, Ting NS, Jye TC, Hotta K, Hatsu M, Fukagawa Y, Shibahara S, Numata K, Kondo S. Bispolides, novel 20-membered ring macrodiolide antibiotics from Microbispora. J Antibiot. 2007;60(3):216–219.
Indananda C, Igarashi Y, Ikeda M, Oikawa T, Thamchaipenet A. Linfuranone A, a new polyketide from plant-derived Microbispora sp. GMKU 363. J Antibiot. 2013;66(11):675–677.
Ivanova V, Kolarova M, Aleksieva K, Gräfe U, Dahse H-M, Laatsch H. Microbiaeratin, a New Natural Indole Alkaloid from a Microbispora aerata Strain, Isolated from Livingston Island, Antarctica. J Prep Biochem Biotech. 2007;37(2):161–168.
Weber T, Blin K, Duddela S, Krug D, Kim HU, Bruccoleri R. AntiSMASH 3.0-a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 2015;43:W237–243.
Medema MH, Takano E, Breitling R. Detecting sequence homology at the gene cluster level with multigeneblast. Mol Biol Evol. 2013;30(5):1218–23.
Shirling EB, Gottlieb D. Methods for characterization of Streptomyces species. Int J Syst Bacteriol. 1966;16(3):313–340.
Kelly KL. Inter-Society Color Council – National Bureau of Standard Color Name Charts Illustrated with Centroid Colors. Washington DC:US Government Printing Office. 1964.
Arai T. Culture Media for Actinomycetes. Tokyo: The Society for Actinomycetes Japan. 1975:1–20.
Williams ST, Cross T. Actinomycetes. In: Booth, C. (Ed.), Methods in Microbiology, vol. 4. Academic Press, London. 1971:295–334.
Gordon RE, Barnett DA, Handerhan JE, Pang CHN. Nocardia coeliaca, Nocardia autotrophica, and the nocardia strain. Int J Syst Bacteriol. 1974;24(1):54–63.
Hasegawa T, Takizawa M, Tanida S. A rapid analysis for chemical grouping of aerobic actinomycetes. J Gen Appl Microbiol. 1983(4);29:319–322.
Komagata K, Suzuki KI. Lipid and cell-wall analysis in bacterial systematics. Methods Microbiol. 1988;19:161–207.
Collins MD, Pirouz T, Goodfellow M, Minnikin DE. Distribution of menaquinones in actinomycetes and Corynebacteria. J Gen Microbiol. 1977;100(2):221–230.
Tamaoka J. Determination of DNA Base Composition. In: Goodfellow, M., O’Donnell, A.G. (Eds.), Chemical Methods in Prokaryotic Systematics. Chichester, John Wiley & Sons, New York. 1994;463–470.
Kittisrisopit S, Pittayakhajonwut P, Tadtong S, Thawai C. Microbispora soli sp. nov., isolated from soil of a hot spring. Int J Syst Evol Microbiol.2018;68(12):3863–3868.
Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1992;173(2):697–703.
Lane DJ. 16S/23S rRNA sequencing. In: Stackebrandt, E., Goodfellow, M. (Eds.), Nucleic Acid Techniques in Bacterial Systematics. Chichester, John Wiley & Sons, New York. 1991;115–175.
Yoon SH, Ha SM, Kwon S, Lim J, Kim Y. Introducing EzBioCloud: A taxonomically united database of 16S rRNA and whole genome assemblies. Int J Syst Evol Microbiol. 2017;67(5):1613–1617.
Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98.
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol. 2013;30(12):2725–2729.
Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39(4):783–791.
Bankevich A, Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19(5):455–477.
Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008;9:75.
Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T. The SEED and the rapid annotation of microbial genomes using subsystems technology (RAST). Nucleic Acids Res. 2014;42:D20–D214.
Richter M, Rosselló-Móra R, Glöckner FO, Peplies J. JSpeciesWS: a web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics. 2016;32(6):929–931.
Meier-Kolthoff JP, Auch AF, Klenk HP, Göker M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinfor. 2013;14:60–73.
Perez C, Paul M, Bazerque P. An antibiotic assay by agar well diffusion method. Acta Biol Med Exp. 1990;15:113–115.
Hayakawa M, Sadakata T, Kajiura T, Nonomura H. New methods for the highly selective isolation of Micromonospora and Microbispora from soil. J Fer Bioeng. 1991;72(5):320–326.
Nakajima Y, Kitpreechavanich V, Suzuki K, Kudo T. Microbispora corallina sp. nov., a new species of the genus Microbispora isolated from Thai soil. Int J Syst Bacteriol. 1999;49(4):1761–1767.
Li C, Zhang Y, Liu C, Wang H, Zhao J. Microbispora bryophytorum sp. nov., an actinomycete isolated from moss (Bryophyta). Int J Syst Evol. Microbiol. 2015;65(Pt_4):1274–1279.
Han C, Liu C, Zhao J, Guo L, Lu C. Microbispora camponoti sp. nov., a novel actinomycete isolated from the cuticle of Camponotus japonicus Mayr. Anto Van Leeuwen. 2016;109(2):215–223.
Boondaeng A, Ishida Y, Tamura T, Tokuyama S, Kitpreechavanich V. Microbispora siamensis sp. nov., a thermotolerant actinomycete isolated from soil. Int J Syst Evol. Microbiol. 2009;59(Pt 12):3136–3139.
Xu, X. X. Wang H-L, Lin H-P, Wang C, Qu Z, Xie Q-Y, Ruan J-S, Hong K. Microbispora hainanensis sp. nov., isolated from rhizosphere soil of Excoecaria agallocha in a mangrove. Int J Syst Evol Microbiol. 2012;62(Pt_10):2430–2434.
Chun J, Oren A, Ventosa A, Christensen H, Arahal DR, da Costa MS. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int J Syst Evol Microbiol. 2018;68(1):461–466.
Wayne LG, Brenner DJ, Colwell RR, Grimont PAD, Kandler O, Krichevsky MI. Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. Int J Syst Bacteriol. 1987;37(4):463–464.
Funabashi M, Funa N, Horinouchi S. Phenolic lipids synthesized by type III polyketide synthase confer penicillin resistance on Streptomyces griseus. J Biol Chem. 2008;283(20):13983–13991.
Giglio S, Jiang J, Saint CP, Cane DE, Monis PT. Isolation and characterization of the gene associated with geosmin production in cyanobacteria. Environ Sci Technol. 2008;42(21):8027–8032.
Becerril A, Álvarez S, Braña AF, Rico S, Díaz M, Santamaría RI. Uncovering production of specialized metabolites by Streptomyces argillaceus: Activation of cryptic biosynthesis gene clusters using nutritional and genetic approaches. PLoS One. 2018;13(5):317–327.
Pan G, Xua Z, Hindraa ZG, Maa M, Yanga D, Zhoua H, Gansemans Y. Discovery of the leinamycin family of natural products by mining actinobacterial genomes. Proc Natl Acad Sci.2017;114(52), E11131–E11140.
Takasaka N, Kaweewan I, Ohnishi-Kameyama M., Kodani S. Isolation of a new antibacterial peptide actinokineosin from Actinokineospora spheciospongiae based on genome mining. Lett Appl Microbiol. 2017;64(2):150–157.
Covington BC, Spraggins JM, Ynigez-Gutierrez AE, Hylton ZB, Bachmann BO. Response of secondary metabolism of hypogean actinobacterial genera to chemical and biological stimuli. Appl Environ Microbiol. 2018;84(19):e01125–18.
Heinzelmann E, Berger S, Müller C, Härtner T, Poralla K, Wohlleben W, Schwartz D. An acyl-CoA dehydrogenase is involved in the formation of the Delta cis3 double bond in the acyl residue of the lipopeptide antibiotic friulimicin in Actinoplanes friuliensis. Microbiology (Reading). 2005;51(Pt 6):1963–1974.
Novakova R, Odnogova Z, Kutas T, Feckova L, Kormanec J. Identification and Characterization of an Indigoidine-like Gene for a Blue Pigment Biosynthesis in Streptomyces aureofaciens CCM 3239. Folia Microbiol. 2010;55(2):119–125.
Downloads
Published
How to Cite
Issue
Section
License
Copyright and Disclaimer
Articles published in this journal are the copyright of Chulabhorn Royal Academy.
The opinions expressed in each article are those of the individual authors and do not necessarily reflect the views of Chulabhorn Royal Academy or any other faculty members of the Academy. The authors are fully responsible for all content in their respective articles. In the event of any errors or inaccuracies, the responsibility lies solely with the individual authors.


