18-Fluoroestradiol (18F-FES) PET Imaging in ER Positive Breast Cancer
Keywords:
FES PET, Fluoroestradiol PET, Estrogen receptor, Breast cancerAbstract
18F-Fluoroestradiol (18F-FES) is an estrogen analogue that has been used for molecular imaging in estrogen receptor (ER) positive breast cancer. In this study are discussed about pharmacokinetic, pharmacodynamic, technical procedure, interpretative criteria, and potential use of 18F-FES in ER positive breast cancer.
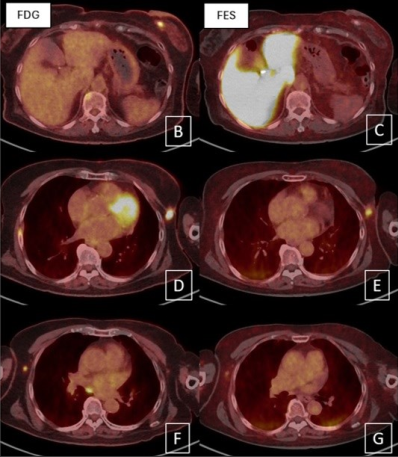
In conclusion, 18F-FES PET imaging in ER positive breast cancer is safe and useful for evaluation of ER expression in the whole body, detection of ER positive lesion, and predict treatment response of hormonal therapy. 18F-FES PET imaging cannot replace but can use as complement to 18F-FDG PET imaging due to heterogeneity of ER expression in some ER positive breast cancer patient.
Downloads
References
Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941-1953. https://doi.org/10.1002/ijc.31937
Blamey RW, Hornmark-Stenstam B, Ball G, et al. ONCOPOOL - a European database for 16,944 cases of breast cancer. Eur J Cancer Oxf Engl 1990. 2010;46(1):56-71. https://doi.org/10.1016/j.ejca.2009.09.009
Cardoso F, Bartlett JMS, Slaets L, et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann Oncol Off J Eur Soc Med Oncol. 2018;29(2):405-417. https://doi.org/10.1093/annonc/mdx651
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Breast Cancer (Version 5.2021).; 2021. Accessed July 8, 2021. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
Allison KH, Hammond MEH, Dowsett M, et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Guideline Update. Arch Pathol Lab Med. 2020;144(5):545-563. https://doi.org/10.5858/arpa.2019-0904-SA
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet Lond Engl. 2015;386(10001):1341-1352. https://doi.org/10.1016/S0140-6736(15)61074-1
Aurilio G, Disalvatore D, Pruneri G, et al. A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. Eur J Cancer Oxf Engl 1990. 2014;50(2):277-289. https://doi.org/10.1016/j.ejca.2013.10.004
Food and Drug Administration (FDA). Drug Trial Snapshot: CERIANNA. FDA; 2020. Accessed July 8, 2021. https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trial-snapshot-cerianna
Venema CM, Apollonio G, Hospers GAP, et al. Recommendations and Technical Aspects of 16α-[18F]Fluoro-17β-Estradiol PET to Image the Estrogen Receptor In Vivo: The Groningen Experience. Clin Nucl Med. 2016;41(11):844-851. https://doi.org/10.1097/RLU.0000000000001347
Peterson LM, Kurland BF, Link JM, et al. Factors influencing the uptake of 18F-fluoroestradiol in patients with estrogen receptor positive breast cancer. Nucl Med Biol. 2011;38(7):969-978. https://doi.org/10.1016/j.nucmedbio.2011.03.002
Linden HM, Kurland BF, Peterson LM, et al. Fluoroestradiol positron emission tomography reveals differences in pharmacodynamics of aromatase inhibitors, tamoxifen, and fulvestrant in patients with metastatic breast cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2011;17(14):4799-4805. https://doi.org/10.1158/1078-0432.CCR-10-3321
Dehdashti F, Mortimer JE, Trinkaus K, et al. PET-based estradiol challenge as a predictive biomarker of response to endocrine therapy in women with estrogen-receptor-positive breast cancer. Breast Cancer Res Treat. 2009;113(3):509-517. https://doi.org/10.1007/s10549-008-9953-0
Peterson LM, Mankoff DA, Lawton T, et al. Quantitative imaging of estrogen receptor expression in breast cancer with PET and 18F-fluoroestradiol. J Nucl Med Off Publ Soc Nucl Med. 2008;49(3):367-374. https://doi.org/10.2967/jnumed.107.047506
Yang Z, Sun Y, Zhang Y, et al. Can fluorine-18 fluoroestradiol positron emission tomography-computed tomography demonstrate the heterogeneity of breast cancer in vivo? Clin Breast Cancer. 2013;13(5):359-363. https://doi.org/10.1016/j.clbc.2013.02.012
Nienhuis HH, van Kruchten M, Elias SG, et al. 18F-Fluoroestradiol Tumor Uptake Is Heterogeneous and Influenced by Site of Metastasis in Breast Cancer Patients. J Nucl Med Off Publ Soc Nucl Med. 2018;59(8):1212-1218. https://doi.org/10.2967/jnumed.117.198846
Seenu V, Sharma A, Kumar R, et al. Evaluation of estrogen expression of breast cancer using 18F-FES PET CT-A novel technique. World J Nucl Med. 2020;19(3):233-239. https://doi.org/10.4103/wjnm.WJNM_71_19
Yang Z, Sun Y, Yao Z, Xue J, Zhang Y, Zhang Y. Increased (18)F-fluoroestradiol uptake in radiation pneumonia. Ann Nucl Med. 2013;27(10):931-934. https://doi.org/10.1007/s12149-013-0761-1
Gupta M, Datta A, Choudhury PS, Dsouza M, Batra U, Mishra A. Can 18F-Fluoroestradiol Positron Emission Tomography Become a New Imaging Standard in the Estrogen Receptor-positive Breast Cancer Patient: A Prospective Comparative Study with 18F-Fluorodeoxyglucose Positron Emission Tomography? World J Nucl Med. 2017;16(2):133-139. https://doi.org/10.4103/1450-1147.203071
Mintun MA, Welch MJ, Siegel BA, et al. Breast cancer: PET imaging of estrogen receptors. Radiology. 1988;169(1):45-48. https://doi.org/10.1148/radiology.169.1.3262228
Kurland BF, Wiggins JR, Coche A, et al. Whole-Body Characterization of Estrogen Receptor Status in Metastatic Breast Cancer with 16α-18F-Fluoro-17β-Estradiol Positron Emission Tomography: Meta-Analysis and Recommendations for Integration into Clinical Applications. The Oncologist. 2020;25(10):835-844. https://doi.org/10.1634/theoncologist.2019-0967
Chae SY, Son HJ, Lee DY, et al. Comparison of diagnostic sensitivity of [18F]fluoroestradiol and [18F]fluorodeoxyglucose positron emission tomography/computed tomography for breast cancer recurrence in patients with a history of estrogen receptor-positive primary breast cancer. EJNMMI Res. 2020;10:54. https://doi.org/10.1186/s13550-020-00643-z
Mortimer JE, Dehdashti F, Siegel BA, Trinkaus K, Katzenellenbogen JA, Welch MJ. Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2001;19(11):2797-2803. https://doi.org/10.1200/JCO.2001.19.11.2797
Linden HM, Stekhova SA, Link JM, et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24(18):2793-2799. https://doi.org/10.1200/JCO.2005.04.3810
Downloads
Published
How to Cite
Issue
Section
License
Copyright and Disclaimer
Articles published in this journal are the copyright of Chulabhorn Royal Academy.
The opinions expressed in each article are those of the individual authors and do not necessarily reflect the views of Chulabhorn Royal Academy or any other faculty members of the Academy. The authors are fully responsible for all content in their respective articles. In the event of any errors or inaccuracies, the responsibility lies solely with the individual authors.


