Myocarditis and pericarditis after mRNA vaccination against COVID-19
Keywords:
mRNA vaccine, myocarditis, pericarditis, myopericarditisAbstract
COVID-19 pandemic has been occurring since 2019. SARS-CoV-2 virus, especially Delta strain, leads to lots of disasters to several countries around the world including Thailand. Therefore, scientists have been trying to develop many platforms of COVID-19 vaccine. To the best of our knowledge, mRNA vaccine is thought to be the most effective platform which can prevent COVID-19 infection and reduce severity of the disease.
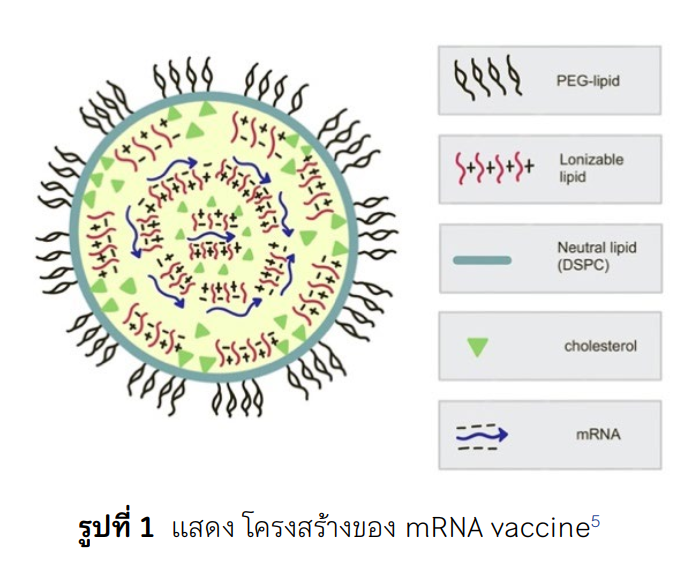
Nowadays, there are 2 types of mRNA vaccine, BNT162b2 by Pfizer company and mRNA-1273 by Moderna company, that have been approved by several organizations. It is recommended to use BNT162b2 and mRNA-1273 in patients more than 12 years old. After significant amount of mRNA vaccine usage worldwide, myocarditis has been reported with the incidence of 16 cases per 1 million doses of vaccinations with unknown mechanism. Moreover, male who are 12-17-year-old possess the highest risk for myocarditis which occur in 56-69 patients per 1 million doses. However, the vaccine can prevent covid-19 infection up to 5,700 patients. Most myocarditis cases are mild in severity and completely reversible with unknown mechanism. There is some assumption of the mechanism of myocarditis which is stimulation and increase in immunity by molecular mimicry or lipid nanoparticle covering the mRNA.
In summary, mRNA vaccine can prevent COVID-19 infection and reduce severity of the disease. Although myocarditis has been reported in some population, benefit of mRNA vaccination is still outweighed this risk. However, the awareness of complication and follow-up after vaccination are still recommended.
Downloads
References
Sharma O, Sultan AA, Ding H, Triggle CR. A review of the progress and challenges of developing a vaccine for COVID-19. Front Immunol. 2020; 11:585354.
Simpson S. What’s in a vaccine? Sci STKE. 2007;2007(367):tw7-tw7.
Callaway E. The race for coronavirus vaccines: a graphical guide. Nature. 2020;580(7805):576- 577. 4. BioNTech: Be unique, treat individualized. Biontech.de. Accessed August 10, 2021. https://biontech.de/hcp-hub/technology-platformsfor-covid-19-vaccines
Izda V, Jeffries MA, Sawalha AH. COVID-19: A review of therapeutic strategies and vaccine candidates. Clin Immunol. 2021; 222(108634): 108634.
Risma KA, Edwards KM, Hummell DS, et al. Potential mechanisms of anaphylaxis to COVID-19 mRNA vaccines. J Allergy Clin Immunol. 2021; 147(6):2075-2082.e2.
Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21(4):195-197.
Schoenmaker L, Witzigmann D, Kulkarni JA, et al. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int J Pharm. 2021; 601 (120586):120586.
Fda.gov. Accessed August 10, 2021. https://www.fda.gov/media/144637/download.
Fda.gov. Accessed August 10, 2021. https://www.fda.gov/media/144413/download.
Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615.
Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416.
Related Biological Products. COVID-19 Vaccine Safety Updates. Fda.gov. Accessed August 10, 2021. https://www.fda.gov/media/150054/download
Segal Y, Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol. 2018;15(6):586-594.
Pirzada A, Mokhtar AT, Moeller AD. COVID-19 and myocarditis: What do we know so far? CJC Open. 2020;2(4):278-285.
VAERS - Report an Adverse Event. Hhs.gov. Accessed August 10, 2021. https://vaers.hhs.gov/reportevent.html
Vaccine Safety Datalink Publications. Cdc.gov. Published June 1, 2021. Accessed August 10, 2021. https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/publications.html
Kim HW, Jenista ER, Wendell DC, et al. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. Published online 2021. https://doi.org/10.1001/jamacardio.2021.2828
Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings -Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70(31):1059-1062.
Luetkens JA, Faron A, Isaak A, et al. Comparison of original and 2018 Lake Louise criteria for diagnosis of acute myocarditis: Results of a validation cohort. Radiol Cardiothorac Imaging. 2019;1(3):e190010.
Larson KF, Ammirati E, Adler ED, et al. Myocarditis after BNT162b2 and mRNA-1273 vaccination. Circulation. Published online 2021. https://doi.org/10.1161/CIRCULATIONAHA.121.055913
Pelliccia A, Sharma S. The 2020 ESC guidelines on sport cardiology. Eur Heart J. 2021;42(1):5
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Chulabhorn Royal Academy

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright and Disclaimer
Articles published in this journal are the copyright of Chulabhorn Royal Academy.
The opinions expressed in each article are those of the individual authors and do not necessarily reflect the views of Chulabhorn Royal Academy or any other faculty members of the Academy. The authors are fully responsible for all content in their respective articles. In the event of any errors or inaccuracies, the responsibility lies solely with the individual authors.


