Radiosynthesis and quality control of [225Ac]AcPSMA-617 for prostate cancer patients by targeted alpha therapy,TAT
Keywords:
Actinium-225(225Ac), Prostate-Specific Membrane Antigen (PSMA), Radiochemical Purity(RCP), Targeted alpha therapy(TAT), LabelingAbstract
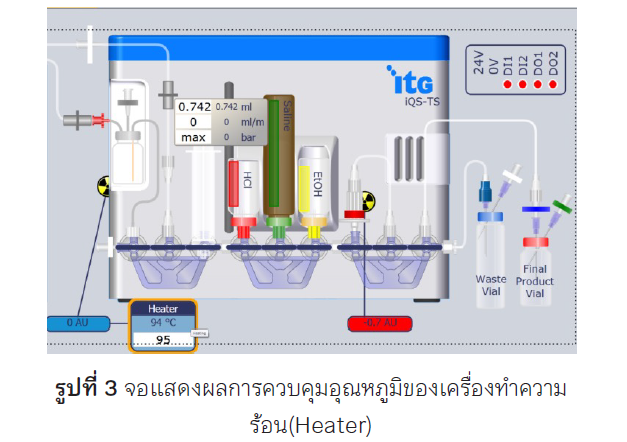
Background: Specific membrane antigen (PSMA) is a highly expressed transmembrane glycoprotein in prostate cancer cells. The radiolabeling of actinium-225 (225Ac) with such peptide is promising as a good radiotracer for use in targeted alpha therapy among patients with metastatic prostate cancer. In this study, the appropriate methods for [225Ac]AcPSMA-617 labeling and quality control with high yield and radiochemical purity is reported. Methods: The radiolabeling of [225Ac]AcPSMA-617 was prepared by adding the PSMA-617 precursor in ascorbate buffer into the 225Ac solution vail. The ratio of the PSMA-617 peptide and 225Ac activity was optimized to 11.0-19.5:1. The mixed solution in reaction vail was heated at 90°C for 10 min. The labelled [225Ac]AcPSMA-617 solution was formulated by adding 0.1 mg/mL of sodium ascorbate in 0.9%NaCl and ethanol(9:1) 10 mL, then transferred through the 0.22 µm filter to the product vial. The quality control of sterile [225Ac]AcPSMA-617 was performed before patient administration. Results: The average radiosynthesis yield of [225Ac]AcPSMA-617 for 14 times by using the above method was 97.28%±2.87%, with the average radiochemical purity of 96.88%±1.30%. Conclusion: The one step of [225Ac]AcPSMA-617 radiolabeling process provides a high yield of synthesis and radiochemical purity (RCP).
Downloads
References
Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer 2018;103:356-387.
Hooijman EL, Chalashkan Y, Ling SW, Kakyargilet FE, Segbers M, Brycgertseufer F, et al. Development of [225Ac]Ac-PSMA-I&T for Targeted Alpha Therapy According to GMP Guidelines for Treatment of mCRPC. Pharmaceutics. 2021;13(5):715.
Ling SW, Blois Ed, Hooijman E, Veldt Avd, Brabander . Advances in 177Lu-PSMA and 225Ac-PSMA Radionuclide Therapy for Metastatic Castration-Resistant Prostate Cancer. Pharmaceutics. 2022;14(10):2166.
Benešová M, Schäfer M, Bauder-Wüst U. Preclinical evaluation of a tailormade DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J Nucl Med. 2015;56:914–920.
Benešová M, Bauder-Wüst U, Schäfer M. Linker modification strategies to control the prostate-specific membrane antigen (PSMA)-targeting and pharmacokinetic properties of DOTA-conjugated PSMA inhibitors. J Med Chem. 2016;59:1761–1775.
Plachta KA, Graves SA, Buatti JM. Prostate-Specific Membrane Antigen (PSMA) Theranostics for Treatment of Oligometastatic Prostate Cancer. Int. J. Mol. Sci. 2021, 22(22), 12095.
Delker A, Fendler WP, Kratochwil C. Dosimetry for 177Lu-DKFZ-PSMA617: a new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:42–51.
Kratochwil C, Bruchertseifer F, Giesel FL, Weis M, Verburg FA, Mottaghy F, et al. 225Ac‑PSMA‑617 for PSMA‑targeted α‑radiation therapy of metastatic castration‑resistant prostatecancer. J Nucl Med. 2016;57:1941-1944.
Kratochwil C, Giesel FL, Bruchertseifer F, Mier W, Apostolidis C, Boll R, et al. 213Bi‑DOTATOC receptor‑targeted alpha‑radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: A first‑in‑human experience. Eur J Nucl Med Mol Imaging. 2014;41: 2106‑2119.
Dauer LT, Williamson MJ, Humm J, Donoghue JO, Ghani R, Awadallah R, et al. Radiation Safety Considerations for the use of 223RaCl2 De in Men with castration – Resistant prostate cancer. Health Phys. 2014;106:494‑504.
Morgenstern A, Apostolidis C, Kratochwil C, Sathekge M, Krolicki L, Bruchertseifer F. An Overview of Targeted Alpha Therapy with 225Actinium and 213Bismuth. Curr Radiopharm. 2018;11:200-208.
Poty S, Francesconi LC, McDevitt MR, Morris MJ Lewis JS. α‑Emitters for radiotherapy: From basic radiochemistry to clinical studies – Part 1. J Nucl Med. 2018;59:878-884.
Kratochwil C, Bruchertseifer F, Rathke H, Hohenfellner M, Giesel FL, Haberkorn U, et al. Targeted α‑therapy of metastatic castration‑resistant prostate cancer with 225Ac‑PSMA‑617: Swimmer‑plot analysis suggests efficacy regarding duration of tumor control. J Nucl Med. 2018;59:795‑802.
Davis IA, Glowienka KA, Boll RA, Deal KA, Brechbiel MW, Stabin M, et al. Comparison of 225actinium chelates: Tissue distribution and radiotoxicity. Nucl Med Biol. 1999;26:581-589.
McDevitt MR, Ma D, Simon J, Frank RK, Scheinberg DA. Design and synthesis of 225Ac radioimmunopharmaceuticals. Appl Radiat Isot. 2002;57:841-847.
Sathege M, Bruchertseifer F, Knoesen O, Reyneke F, Lawal I, Lengana T, et al. 225Ac‑PSMA‑617 in chemotherapy‑naïve patients with advanced prostate cancer: A pilot study. Eur J Nucl Med Mol Imaging. 2019;46:129-138.
Thakral P, Simecek J, Marx S, Kumari J, Pant V, Sen IB. In-House Preparation and Quality Control of Ac-225 Prostate-Specific Membrane Antigen-617 for the Targeted Alpha Therapy of Castration-Resistant Prostate Carcinoma. Indian J Nucl Med. 2021;36(2):114-119.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Chulabhorn Royal Academy

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright and Disclaimer
Articles published in this journal are the copyright of Chulabhorn Royal Academy.
The opinions expressed in each article are those of the individual authors and do not necessarily reflect the views of Chulabhorn Royal Academy or any other faculty members of the Academy. The authors are fully responsible for all content in their respective articles. In the event of any errors or inaccuracies, the responsibility lies solely with the individual authors.


