Bell’s palsy after inactivated COVID-19 vaccination in an adolescent: A case report
Keywords:
Bell’s palsy, Facial palsy, COVID-19, BBIBP-CorV, Inactivated vaccine, High power laser therapyAbstract
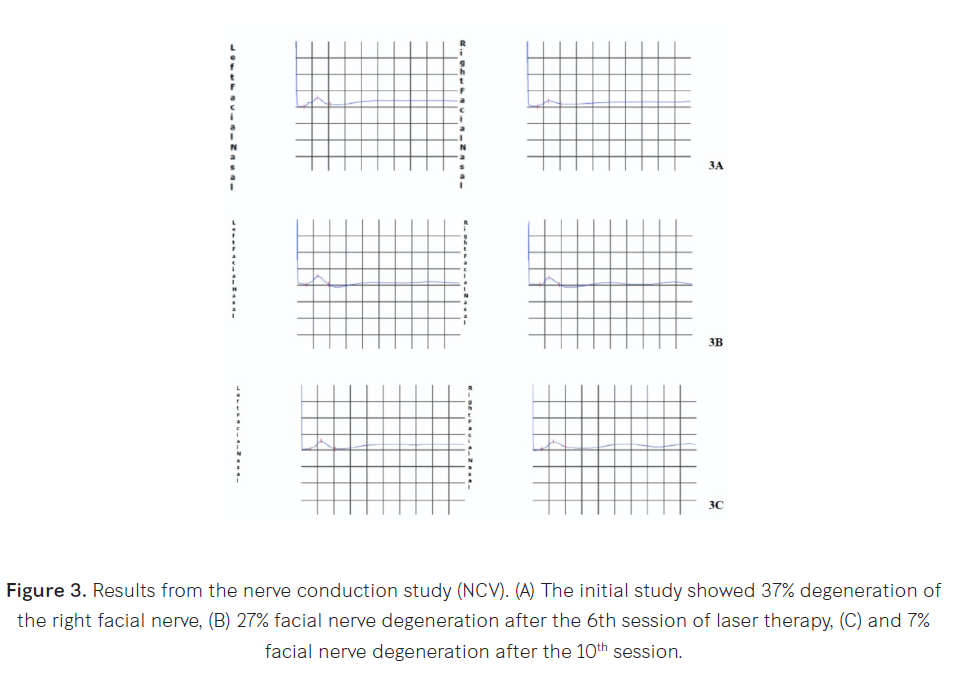
Bell’s palsy has been reported following COVID-19 vaccination in adults. Here, we report a case of an adolescent male with unilateral peripheral facial palsy after receiving the BBIBP-CorV vaccine. An electrodiagnostic study confirmed the diagnosis. The patient made a complete recovery after medical and rehabilitation treatment. Objectives: This is the first case report of Bell’s palsy following the administration of an inactivated COVID-19 vaccine in an adolescent. Study design: A case report. Setting: Chulabhorn Hospital, Lak Si, Bangkok, Thailand. Subject: An adolescent male with unilateral peripheral facial palsy after receiving the BBIBP-CorV vaccine. Methods: We reviewed the patient’s medical record and electrodiagnostic report. Results: A 14-year-old, previously healthy, adolescent male received his 1st dose of inactivated BBIBP-CorV (Sinopharm) COVID-19 vaccine. Five days later, he developed right peripheral facial palsy. He was diagnosed with Bell’s palsy. Clinical signs and symptoms combined with initial and follow-up electrodiagnostic studies confirmed the diagnosis. He was treated for 5 days with oral prednisolone and vitamin B-complex. He received eye care with artificial tears and underwent a rehabilitation program using the bio-stimulation mode of high-power laser therapy (MLS®) combined with standard physiotherapy. Conclusions: Bell’s palsy might be an adverse event associated with the inactivated BBIBP-CorV COVID-19 vaccine in adolescents.
Downloads
References
Gilden DH. Clinical practice. Bell's palsy. N Engl J Med. 2004;351(13):1323–1331.
Singhi P and Jain V. Bell’s palsy in children. Semin Pediatr Neurol. 2003;10(4):289–297.
Karalok ZS, Taskin BD, Ozturk Z, et al. Childhood peripheral facial palsy. Childs Nerv Syst. 2018;34(5):911–917.
Baugh RF, Basura GJ, Ishii LE, et al. Clinical practice guideline: Bell’s palsy. Otolaryngol Head Neck Surg. 2013;149(3 suppl):S1–S27.
Dumitru D, Amato A, Zwarts M. Electrodiagnostic medicine. second edition. Philadelphia: Hanley&Belfus; 2002.
Pitaro J, Waissbluth S, Daniel SJ. Do children with Bell’ palsy benefit from steroid treatment? A systematic review. Int J Pediatr Otorhinolaryngol. 2012;76(7):921–926.
Yoo HW, Yoon L, Kim HY, et al. Comparison of conservative therapy and steroid therapy for Bell’s palsy in children. Korean J Pediatr. 2018;61(10):332–337.
Ismail AQ, Alake O, Kallappa C. Do oral steroids aid recovery in children with Bell’s palsy? J Child Neurol. 2014;29(10):NP96–NP97.
Rowhani-Rahbar A, Klein NP, Lewis N, et al. Immunization and Bell’s palsy in children: a case-centered analysis. Am J Epidemiol. 2012;175(9):878–885.
Bardage C, Persson I, Örtqvist Å, et al. Neurological and autoimmune disorders after vaccination against pandemic influenza A (H1N1) with a monovalent adjuvanted vaccine: population based cohort study in Stockholm, Sweden. BMJ. 2011;343:d5956.
Wan EYF, Chui CSL, Lai FTT, et al. Bell’s palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2022;22(1):64–72.
Thonginnetra S, Tawinprai K, Niemsorn K, et al. Safety after BBIBP-CorV (Sinopharm) COVID-19 vaccine in adolescents aged 10-17 years in Thailand. Vaccines. 2022;10:1765.
Preston DC and Shapiro BE. Electromyography and neuromuscular disorders: Clinical-electrophysiologic correlations. third edition. London, UK: Elsevier Saunders; 2013.
Dumitru D, Amato A, Zwarts M. Electrodiagnostic medicine. second edition. Philadelphia: Hanley&Belfus; 2002.
Nishizawa Y, Hoshina Y, Baker V. Bell’s palsy following the Ad26.COV2.S COVID-19 vaccination. QJM. 2021;114(9):657–658.
Wijnans L, Dodd CN, Weibel D, Sturkenboom M. Bell’s palsy and influenza (H1N1) pdm09 containing vaccines: A self-controlled case series. PLoS One. 2017;12(5): e0175539.
Sato K, Mano T, Niimi Y, et al. Facial nerve palsy following the administration of COVID-19 mRNA vaccines: Analysis of a self-reporting database. Int J Infect Dis. 2021;111:310–312.
Hashmi JT, Huang YY, Osmani BZ, et al. Role of low-level laser therapy in neurorehabilitation. PM&R. 2010;2(12 Suppl):S292–S305.
Santamato A, Solfrizzi V, Panza F, et al. Short-term effects of high-intensity laser therapy versus ultrasound therapy in the treatment of people with subacromial impingement syndrome: a randomized clinical trial. Phys Ther. 2009;89(7):643–652.
Mester A. Laser biostimulation. Photomed Laser Surg. 2013;31(6):237–239.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Chulabhorn Royal Academy

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright and Disclaimer
Articles published in this journal are the copyright of Chulabhorn Royal Academy.
The opinions expressed in each article are those of the individual authors and do not necessarily reflect the views of Chulabhorn Royal Academy or any other faculty members of the Academy. The authors are fully responsible for all content in their respective articles. In the event of any errors or inaccuracies, the responsibility lies solely with the individual authors.


