Effects of proprotein convertase subtilisin/kexin type9 inhibitors on salivary glands of obese rats induced by high-fat diet consumption
Main Article Content
Abstract
Background and Objective(s): Obesity could alter cellular metabolism, leading to insulin resistance and increased vulnerability to oxidative stress, inflammation, and impairment of peripheral organs. Atorvastatin and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors have been approved as lipid-lowering agents for patients with dyslipidemia and have shown potential in enhancing metabolic, brain, and cognitive functions in obese patients. However, the impact of atorvastatin and PCSK9 inhibitors on salivary glands in an obesity model is still insufficiently explored.
Material and Methods: Twenty-four female Wistar rats (Rattus norvegicus) were divided into two groups: those fed a normal diet (ND) and those fed a high-fat diet (HFD), for 16 weeks. During the final four weeks of the study, ND-fed rats received subcutaneous injections of normal saline solution (NDV), while HFD-fed rats were further subdivided intothree treatment groups: normal saline solution (HFV), atorvastatin (40 mg/kg/day; HFA), and PCSK9 inhibitor (4 mg/kg/day; HFP). Submandibular salivary glands were removed to evaluate oxidative stress, inflammation, and apoptosis.
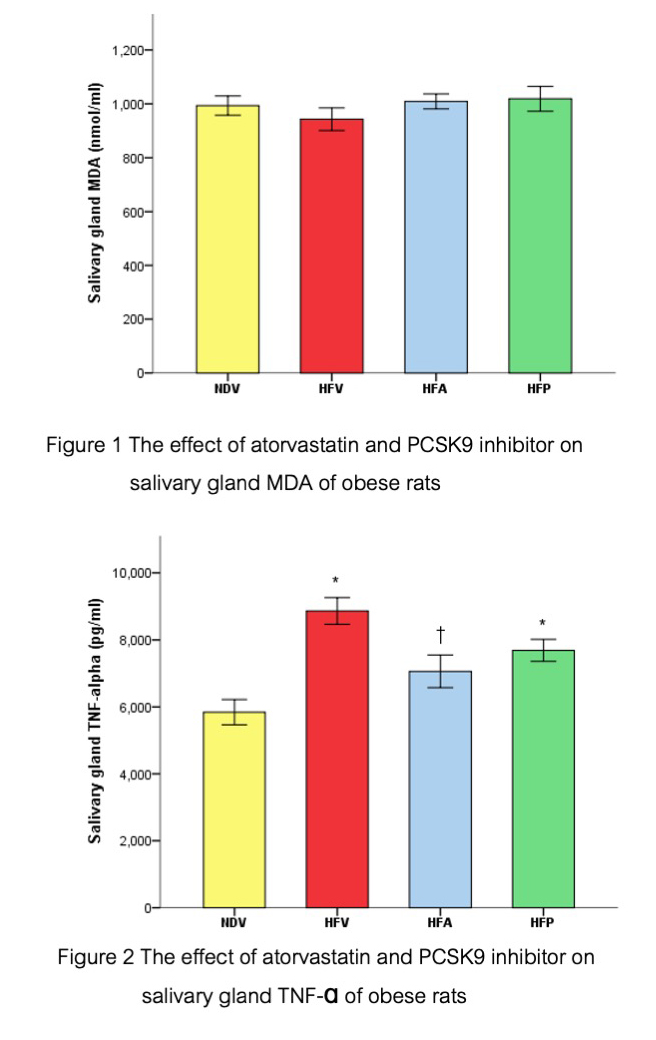
Results: Consumption of the HFD led to obesity and damage to the salivary glands, evidenced by increased inflammation and reduced anti-apoptotic expression. Conversely, treatment with atorvastatin and PCSK9 inhibitors improved salivary gland conditions in obese rats, as shown by decreased inflammation and enhanced anti-apoptotic expression.
Conclusion: Atorvastatin and PCSK9 inhibitors prevented salivary gland injury associated with obesity.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Hruby A, Hu FB. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics. 2015 Jul;33(7):673-689. doi: 10.1007/s40273-014-0243-x.
Bai L, Li H. Innate immune regulatory networks in hepatic lipid metabolism. J Mol Med (Berl). 2019 May;97(5):593-604. doi: 10.1007/s00109-019-01765-1.
Dale CE, Fatemifar G, Palmer TM, White J, Prieto-Merino D, Zabaneh D, et al. Causal Associations of Adiposity and Body Fat Distribution With Coronary Heart Disease, Stroke Subtypes, and Type 2 Diabetes Mellitus: A Mendelian Randomization Analysis. Circulation. 2017 Jun;135(24):2373-2388. doi: 10.1161/CIRCULATIONAHA.116.026560.
Amput P, Palee S, Arunsak B, Pratchayasakul W, Jaiwongkam T, Chattipakorn S, et al. PCSK9 inhibitor and atorvastatin improve cardiac and motochondrial function in obese-insulin resistant rats. J Am Coll Cardiol. 2019;73(9 Suppl 1):1327.
Ittichaicharoen J, Chattipakorn N, Chattipakorn SC. Is salivary gland function altered in noninsulin-dependent diabetes mellitus and obesity–insulin resistance? Arch Oral Biol. 2016 Apr;64:61-71. doi: 10.1016/j.archoralbio.2016.01.002.
Pintana H, Apaijai N, Chattipakorn N, Chattipakorn SC. DPP-4 inhibitors improve cognition and brain mitochondrial function of insulin-resistant rats. J Endocrinol. 2013 May;218(1):1-11. doi: 10.1530/JOE-12-0521.
Alhajj M, Babos M. Physiology, Salivation. [Updated 2023 Jul 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542251/
Ghannam MG, Singh P. Anatomy, Head and Neck, Salivary Glands. [Updated 2023 May 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538325/
Roa I, Del Sol M. Obesity, salivary glands and oral pathology. Colomb Med (Cali). 2018 Dec;49(4):280-287. doi: 10.25100/cm.v49i3.3919.
Laufs U, Karmann B, Pittrow D. Atorvastatin treatment and LDL cholesterol target attainment in patients at very high cardiovascular risk. Clin Res Cardiol. 2016 Sep;105(9):783-790. doi: 10.1007/s00392-016-0991-z.
Ramkumar S, Raghunath A, Raghunath S. Statin Therapy: Review of Safety and Potential Side Effects. Acta Cardiol Sin. 2016 Nov;32(6):631-639. doi: 10.6515/acs20160611a.
Chen L, Chen XW, Huang X, Song BL, Wang Y, Wang Y. Regulation of glucose and lipid metabolism in health and disease. Sci China Life Sci. 2019 Nov;62(11):1420-1458. doi: 10.1007/s11427-019-1563-3.
Pengrattanachot N, Cherngwelling R, Jaikumkao K, Pongchaidecha A, Thongnak L, Swe MT, et al. Atorvastatin attenuates obese-induced kidney injury and impaired renal organic anion transporter 3 function through inhibition of oxidative stress and inflammation. Biochim Biophys Acta Mol Basis Dis. 2020 Jun;1866(6):165741. doi: 10.1016/j.bbadis.2020.165741.
McIver LA, Siddique MS. Atorvastatin. [Updated 2022 Nov 13]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430779/
Arunsak B, Pratchayasakul W, Amput P, Chattipakorn K, Tosukhowong T, Kerdphoo S, et al. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor exerts greater efficacy than atorvastatin on improvement of brain function and cognition in obese rats. Arch Biochem Biophys. 2020 Aug;689:108470. doi: 10.1016/j.abb.2020.108470.
Bergeron N, Phan BA, Ding Y, Fong A, Krauss RM. Proprotein convertase subtilisin/kexin type 9 inhibition: a new therapeutic mechanism for reducing cardiovascular disease risk. Circulation. 2015 Oct;132(17):1648-1666. doi: 10.1161/CIRCULATIONAHA.115.016080.
Pratchayasakul W, Kerdphoo S, Petsophonsakul P, Pongchaidecha A, Chattipakorn N, Chattipakorn SC. Effects of high-fat diet on insulin receptor function in rat hippocampus and the level of neuronal corticosterone. Life Sci. 2011 Mar;88(13-14):619-627. doi: 10.1016/j.lfs.2011.02.003.
Pipatpiboon N, Pintana H, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. DPP4-inhibitor improves neuronal insulin receptor function, brain mitochondrial function and cognitive function in rats with insulin resistance induced by high-fat diet consumption. Eur J Neurosci. 2013 Mar;37(5):839-849. doi: 10.1111/ejn.12088.
Sripetchwandee J, Pipatpiboon N, Pratchayasakul W, Chattipakorn N, Chattipakorn S. DPP-4 Inhibitor and PPARγ Agonist Restore the Loss of CA1 Dendritic Spines in Obese Insulin-resistant Rats. Arch Med Res. 2014 Oct;45(7):547-552. doi: 10.1016/j.arcmed.2014.09.002.
Suzuki M, Kakuta H, Takahashi A, Shimano H, Tada-Iida K, Yokoo T, et al. Effects of atorvastatin on glucose metabolism and insulin resistance in KK/Ay mice. J Atheroscler Thromb. 2005;12(2):77-84. doi: 10.5551/jat.12.77.
Ittichaicharoen J, Apaijai N, Tanajak P, Sa-nguanmoo P, Chattipakorn N, Chattipakorn S. Dipeptidyl peptidase-4 inhibitor enhances restoration of salivary glands impaired by obese-insulin resistance. Arch Oral Biol. 2018 Jan;85:148-153. doi: 10.1016/j.archoralbio.2017.10.015.
Denning TL, Takaishi H, Crowe SE, Boldogh I, Jevnikar A, Ernst PB. Oxidative stress induces the expression of Fas and Fas ligand and apoptosis in murine intestinal epithelial cells. Free Radic Biol Med. 2002 Dec;33(12):1641-1650. doi: 10.1016/s0891-5849(02)01141-3.
Suzuki M, Aoshiba K, Nagai A. Oxidative stress increases Fas ligand expression in endothelial cells. J Inflamm (Lond). 2006 Jul;3:11. doi: 10.1186/1476-9255-3-11.