Healing of dehiscence defect around implants using β-tricalcium phosphate/calcium sulfate versus conventional guided bone regeneration: a pilot study
Main Article Content
Abstract
Objectives: To evaluate and compare the healing outcomes of buccal dehiscence defects around dental implants using beta-tricalcium phosphate calcium sulfate (β-TCP/CS) and deproteinized bovine bone mineral mixed with a collagen membrane (DBBM/CM).
Materials and Methods: Two distinct groups were established for the study: the β-TCP/CS group (n=5) and the DBBM/CS group (n=5). The clinical evaluation was assessed by comparing the percentage of total volume alteration between pre-operative and 6 months post-operative 3D analyses. Radiological evaluations were conducted to assess bone graft thickness at the implant placement date and after a 6-month healing period.
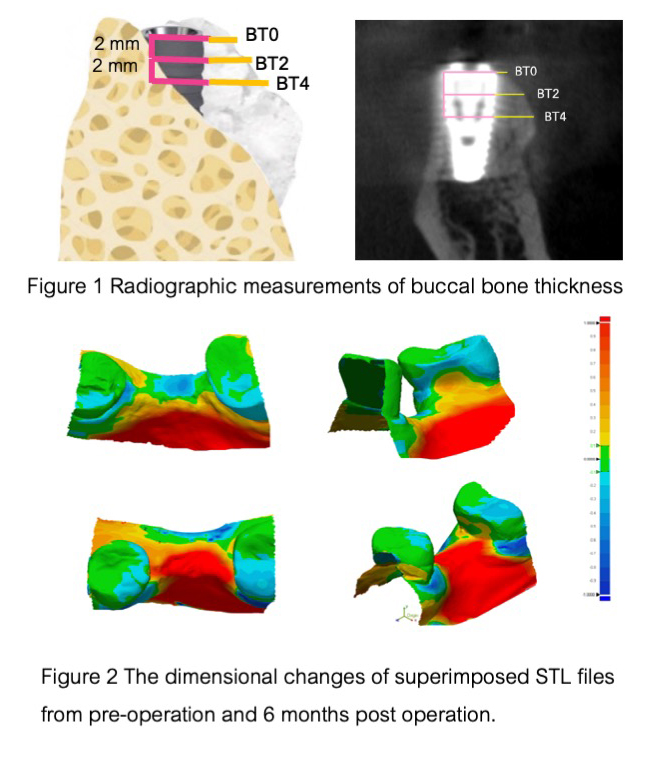
Results: Both groups exhibit uneventful defect healing and 100% implant survival after 6-month healing period. Regarding the total volume alteration at the grafted site, β-TCP/CS exhibited 5.10%±2.32 alteration, while DBBM/ CM exhibited 6.04%±3.07 alteration and no statistically significant difference (p>0.05) was observed. In Cone Beam Computed Tomography radiographic measurement, buccal bone thickness at 6 months at the platform level (BT0), 2mm (BT2), and 4mm (BT4) from the platform level after 6 months were 0.65±0.39 mm, 1.25±0.75 mm, and 2.79±0.37 mm in the β-TCP/CS group. For the DBBM/CM group, the corresponding values were 0.93±0.38 mm, 2.73±0.39 mm, and 3.22±0.99 mm, respectively. A statistically significant difference was observed between the two groups at BT2 (p=0.004). The percentage of bone graft thickness alteration at various points (%ΔBT0, %ΔBT2, and %ΔBT4) in the β-TCP/CS group showed 65.38%±20.44,58.49%±26.86 and 33.10%±18.28 respectively, when compared to the DBBM/CM group, where the corresponding values were 48.71%±26.03, 19.78%±9.29 and 8.72%±7.34 with a significant difference at %ΔBT2(p=0.016)
and %ΔBT4(p=0.024).
Conclusions: β-TCP/CS demonstrated comparable overall clinical healing of small dehiscence defects around implants. However, β-TCP/CS resulted in greater graft reduction and less bone graft stabilization at 6-month follow-up.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Tan WL, Wong TLT, Wong MCM, Lang NP. A systematic review of post-extractional alveolar hard and soft tissue
dimensional changes in humans. Clinical Oral Implants Res. 2012;23:1-21.doi10.1111/j.1600-0501.2011.02375.
Bornstein MM, Halbritter S, Harnisch H, Weber H-P, Buser DJIjoo, implants m. A retrospective analysis of patients
referred for implant placement to a specialty clinic: indications, surgical procedures, and early failures.
International journal of oral & maxillofacial implants.2008;23(6).
Mayfield L, Nobréus N, Attström R, Linde AJCoir. Guided bone regeneration in dental implant treatment using a
bioabsorbable membrane. Clinical oral implants research.1997;8(1):10-7.
Khojasteh A, Kheiri L, Motamedian SR, Khoshkam VJAoms. Guided bone regeneration for the reconstruction of
alveolar bone defects. Ann of maxillofac surg.2017;7(2):263.doi:10.4103/ams.ams_76_17.
Elgali I, Omar O, Dahlin C, Thomsen PJEjoos. Guided bone regeneration: materials and biological mechanisms
revisited. European Journal of Oral Sciences 2017;125(5):315-37.doi: 10.1111/eos.12364.
Thoma DS, Bienz SP, Figuero E, Jung RE, Sanz‐Martín IJJocp. Efficacy of lateral bone augmentation performed
simultaneously with dental implant placement: A systematic review and meta‐analysis. Journal of Clinical
Periodontology.2019;46:257-76.doi: 10.1111/jcpe.13050.
Haugen HJ, Lyngstadaas SP, Rossi F, Perale GJJoCP. Bone grafts: which is the ideal biomaterial?.Journal of
Clinical Periodontology. 2019;46:92-102.doi: 10.1111/jcpe.13058.
Podaropoulos L, Veis AA, Papadimitriou S, Alexandridis C, Kalyvas DJJoOI. Bone regeneration using btricalcium phosphate in a calcium sulfate matrix. J Oral Implantol. 2009;35(1):28-36.doi: 10.1563/1548-1336-
1.28
Stronger eG. EthOss Grow Stronger (internet). UK: Ethoss Regeneration Ltd; [cited 2021 June 6].
Fairbairn P, Leventis M, Mangham C, Horowitz R. Alveolar Ridge Preservation Using a Novel Synthetic Grafting
Material: A Case with Two-Year Follow-Up. Case Rep Dent. 2018;.doi:10.1155/2018/6412806.
Baranes D, Kurtzman GM. Biphasic Calcium Sulfate as an Alternative Grafting Material in Various Dental
Applications. J Oral Implantol. 2019;45(3):247-55.doi: 10.1563/aaid-joi-D-18-00306.
Leventis M, Fairbairn P, Mangham C, Galanos A, Vasiliadis O, Papavasileiou D, et al. Bone Healing in Rabbit
Calvaria Defects Using a Synthetic Bone Substitute: A Histological and Micro-CT Comparative Study. Materials.
;11(10).doi: 10.3390/ma11102004.
Fairbairn P, Leventis MJIJoD. Protocol for bone augmentation with simultaneous early implant placement: a
retrospective multicenter clinical study. International journal of dentistry. 2015;2015.doi:10.1155/2015/589135.
Al Khadher AA, Abdelsameaa SE, Ahmed WMJMJoD. Ethoss versus mineralized plasmatic matrix for maxillary
lateral sinus lifting with simultaneous implant placement. Mansoura Journal of Dental. 2022;9:38-44.
Basler T, Naenni N, Schneider D, Hämmerle CH, Jung RE, Thoma DSJCoir. Randomized controlled clinical study
assessing two membranes for guided bone regeneration of peri‐implant bone defects: 3‐year results. Clinical
oral implants research. 2018;29(5):499-507.doi: 10.1111/clr.13147.
Mir‐Mari J, Wui H, Jung RE, Hämmerle CH, Benic GIJCOIR. Influence of blinded wound closure on the volume
stability of different GBR materials: an in vitro cone‐beam computed tomographic examination. Clinical Oral
Implants Research,2016;27(2):258-65.doi: 10.1111/clr.12590.
Jeong J, Kim JH, Shim JH, Hwang NS, Heo CY. Bioactive calcium phosphate materials and applications in bone
regeneration. Biomater Res. 2019;23:4.
Bohner M, Santoni BLG, Döbelin NJAb. β-tricalcium phosphate for bone substitution: Synthesis and properties.
;113:23-41. Acta biomaterialia, doi:10.1016/j.actbio.2020.06.022
Kokubo T. Bioceramics and their clinical applications: Elsevier; 2008.
Ricci JL WM, Mamidwar S, Alexander H. . Calcium sulfate. In Bioceramics and their Clinical Applications
Woodhead Publishing.; 2008 Jan 1 p. 302-25.
Nooh N, Ramalingam S, Al-Kindi M, Al-Rasheed A, Al-Hamdan KS, Al-Hezaimi KJIJoP, et al. Real-Time
Assessment of Guided Bone Regeneration in Standardized Calvarial Defects in Rats Using Bio-Oss With and
Without Collagen Membrane: An In Vivo Microcomputed Tomographic and Histologic Experiment. International
Journal of Periodontics & Restorative Dentistry. 2016;36.
Yahav A, Kurtzman GM, Katzap M, Dudek D, Baranes DJDc. Bone regeneration: Properties and clinical
applications of biphasic calcium sulfate. Dental clinics. 2020;64(2):453-72.doi: 10.1016/j.cden.2019.12.006.
Leventis M, Agrogiannis G, Fairbairn P, Vasiliadis O, Papavasileiou D, Theodoropoulou E, et al. Evaluation of an
In Situ Hardening beta-Tricalcium Phosphate Graft Material for Alveolar Ridge Preservation. A Histomorphometric
Animal Study in Pigs. Dent J (Basel). 2018;6(3).doi: 10.3390/dj6030027
Fu J-H, Oh T-J, Benavides E, Rudek I, Wang H-L. A randomized clinical trial evaluating the efficacy of the
sandwich bone augmentation technique in increasing buccal bone thickness during implant placement surgery.
Clinical Oral Implants Research. 2014;25(4):458-67.doi: 10.1111/clr.12171.
Jung RE, Herzog M, Wolleb K, Ramel CF, Thoma DS, Hammerle CH. A randomized controlled clinical trial
comparing small buccal dehiscence defects around dental implants treated with guided bone regeneration or
left for spontaneous healing. Clin Oral Implants Res. 2017;28(3):348-54.doi: 10.1111/clr.12806