Mitochondrial dysfunction in the salivary glands of type 1 diabetes mellitus rats: effects of insulin and atorvastatin treatment
Main Article Content
Abstract
Background and Objective: Type 1 Diabetes mellitus (T1DM) involves pancreatic beta-cell dysfunction and inadequate insulin production, leading to disrupted blood glucose levels and subsequent organ dysfunction. The aim of this study was to examine the influence of insulin and statins on oxidative stress and mitochondrial dysfunction in the salivary glands of streptozotocin-induced diabetic rats.
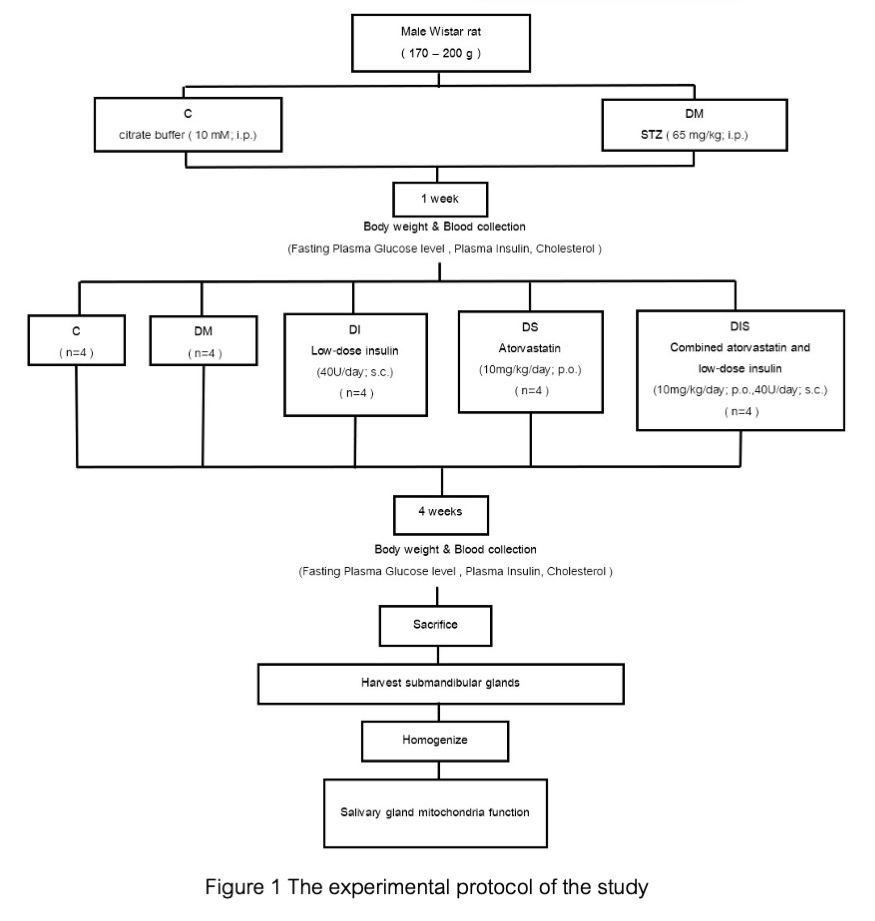
Material and Methods: Twenty male rats were divided into control (n=4) and experimental groups (n=16) of streptozotocin-induced diabetic rats. Diabetic rats were further divided into four groups (n=4/group) receiving vehicle, low-dose atorvastatin, low-dose insulin, or combined drugs for 4 weeks. Metabolic parameters were assessed via blood samples. Mitochondrial function in submandibular salivary glands was evaluated for reactive oxidative stress (ROS), mitochondrial membrane potential, and swelling analysis.
Results: Streptozotocin-induced T1DM rats displayed hyperglycemia, indicating heightened mitochondrial dysfunction in salivary glands. Mitochondrial ROS production and membrane depolarization were significantly elevated compared to normal rats (p<0.05). Atorvastatin, insulin, and combination therapy similarly mitigated mitochondrial dysfunction in induced T1DM rats (p<0.05). All three treatment groups significantly reduced plasma glucose, while combined therapy was the most effective.
Conclusion: Combined drug therapy demonstrated the highest efficacy in improving metabolic parameters. Atorvastatin, insulin, and combined therapy were equally effective in mitigating mitochondrial dysfunction in the salivary glands of induced T1DM rats. These findings suggest the potential of combination therapy for T1DM management. Further investigations are needed to understand their impact on salivary gland function and implications for oral health and overall well-being in individuals with T1DM.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Ma Z, Li L, Livingston MJ, Zhang D, Mi Q, Zhang M, et al. p53/microRNA-214/ULK1 axis impairs renal tubular autophagy in diabetic kidney disease. J Clin Invest. 2020 Sep;130(9):5011-5026. doi: 10.1172/JCI135536.
Pratchayasakul W, Thongnak LO, Chattipakorn K, Lungaphin A, Pongchaidecha A, Satjaritanun P, et al. Atorvastatin and insulin equally mitigate brain pathology in diabetic rats. Toxicol Appl Pharmacol. 2018 Mar;342:79-85. doi: 10.1016/j.taap.2018.01.021.
Nakaya H, Takeda Y, Tohse N, Kanno M. Mechanism of the membrane depolarization induced by oxidative stress in guinea-pig ventricular cells. J Mol Cell Cardiol. 1992 May;24(5):523-534. doi: 10.1016/0022-2828(92)91841-r.
Peng TI, Jou MJ. Mitochondrial swelling and generation of reactive oxygen species induced by photoirradiation are heterogeneously distributed. Ann N Y Acad Sci. 2004 Apr;1011:112-122. doi: 10.1007/978-3-662-41088-2_12.
Murphy MP. Mitochondrial dysfunction indirectly elevates ROS production by the endoplasmic reticulum. Cell Metab. 2013 Aug;18(2):145-146. doi: 10.1016/j.cmet.2013.07.006.
Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014 May;24(10):R453-462. doi: 10.1016/j.cub.2014.03.034.
Edblad E, Lundin SA, Sjödin B, Aman J. Caries and salivary status in young adults with type 1 diabetes. Swed Dent J. 2001;25(2):53-60.
Moore PA, Guggenheimer J, Etzel KR, Weyant RJ, Orchard T. Type 1 diabetes mellitus, xerostomia, and salivary flow rates. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001 Sep;92(3):281-291. doi: 10.1067/moe.2001.117815.
Alves C, Menezes R, Brandão M. Salivary flow and dental caries in Brazilian youth with type 1 diabetes mellitus. Indian J Dent Res. 2012 Nov-Dec;23(6):758-762. doi: 10.4103/0970-9290.111254.
Knaś M, Maciejczyk M, Daniszewska I, Klimiuk A, Matczuk J, Kołodziej U, et al. Oxidative damage to the salivary glands of rats with Streptozotocin-induced diabetes-temporal study: oxidative stress and diabetic salivary glands. J Diabetes Res. 2016;2016:4583742. doi: 10.1155/2016/4583742.
Xu L, Yang X, Chen J, Ge X, Qin Q, Zhu H, et al. Simvastatin attenuates radiation-induced salivary gland dysfunction in mice. Drug Des Devel Ther. 2016 Jul;10:2271-2278. doi: 10.2147/DDDT.S105809.
Herzog RI, Sherwin RS, Rothman DL. Insulin-induced hypoglycemia and its effect on the brain: unraveling metabolism by in vivo nuclear magnetic resonance. Diabetes. 2011 Jul;60(7):1856-1858. doi: 10.2337/db11-0498.
McCall AL. Insulin therapy and hypoglycemia. Endocrinol Metab Clin North Am. 2012 Mar;41(1):57-87. doi: 10.1016/j.ecl.2012.03.001.
Heller SR, Peyrot M, Oates SK, Taylor AD. Hypoglycemia in patient with type 2 diabetes treated with insulin: it can happen. BMJ Open Diabetes Res Care. 2020 Jun;8(1):e001194. doi: 10.1136/bmjdrc-2020-001194.
Alotaibi A, Sultan BA, Buzeid R, Almutairi M, Alghamdi E, Aldhaeefi M, et al. An overview of insulin therapy in pharmacotherapy of diabetes mellitus type I. Int J Community Med Public Health. 2018 Mar;5(3):834-838. doi: 10.18203/2394-6040.ijcmph20180418.
Hatting M, Tavares CDJ, Sharabi K, Rines AK, Puigserver P. Insulin regulation of gluconeogenesis. Ann N Y Acad Sci. 2018 Jan;1411(1):21-35. doi: 10.1111/nyas.13435.
Parida S, Swain TR, Routray SN, Maiti R. Effect of atorvastatin on glycaemic parameters in normoglycaemic and prediabetic subjects: A prospective, panel study. J Clin Diagn Res. 2017 Feb;11(2):FC04-FC09. doi: 10.7860/JCDR/2017/23741.9427.
Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016 Apr;23(4):591-601. doi: 10.1016/j.cmet.2016.02.005.
Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes--causes, effects and coping strategies. Diabetes Obes Metab. 2007 Nov;9(6):799-812. doi: 10.1111/j.1463-1326.2006.00686.x.
Anyanwagu U, Mamza J, Donnelly R, Idris I. Effects of background statin therapy on glycemic response and cardiovascular events following initiation of insulin therapy in type 2 diabetes: a large UK cohort study. Cardiovasc Diabetol. 2017 Aug;16(1):107. doi: 10.1186/s12933-017-0587-6.
Kawahito S, Kitahata H, Oshita S. Problems associated with glucose toxicity: role of hyperglycemia-induced oxidative stress. World J Gastroenterol. 2009 Sep;15(33):4137-4142. doi: 10.3748/wjg.15.4137.
Yan LJ. Pathogenesis of chronic hyperglycemia: from reductive stress to oxidative stress. J Diabetes Res. 2014;2014:137919. doi: 10.1155/2014/137919.
Fleury C, Mignotte B, Vayssière JL. Mitochondrial reactive oxygen species in cell death signaling. Biochimie. 2002 Feb-Mar;84(2-3):131-141. doi: 10.1016/s0300-9084(02)01369-x.
Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007 May;12(5):913-922. doi: 10.1007/s10495-007-0756-2.
Kamogashira T, Fujimoto C, Yamasoba T. Reactive oxygen species, apoptosis, and mitochondrial dysfunction in hearing loss. BioMed Res Int. 2015;2015:617207. doi: 10.1155/2015/617207.
Hancock JT, Desikan R, Neill SJ. Role of reactive oxygen species in cell signalling pathways. Biochem Soc Trans. 2001 May;29(Pt 2):345-350. doi: 10.1042/0300-5127:0290345.
Bokoch GM, Diebold BA. Current molecular models for NADPH oxidase regulation by Rac GTPase. Blood. 2002 Oct;100(8):2692-2696. doi: 10.1182/blood-2002-04-1149.
Cherian DA, Peter T, Narayanan A, Madhavan SS, Achammada S, Vynat GP. Malondialdehyde as a marker of oxidative stress in periodontitis patients. J Pharm Bioallied Sci. 2019 May;11(Suppl 2):S297-S300. doi: 10.4103/JPBS.JPBS_17_19.