Effect of mineral trioxide aggregate mixed with Thai propolis extract on matrix metalloproteinase-2 expression in inflamed human dental pulp cells
Main Article Content
Abstract
Objective: To investigate the inhibition activity of mineral trioxide aggregate (ProRoot®MTA) mixed with Thai propolis extract on the expression of Matrix Metalloproteinase-2 (MMP-2) in IL-1β-stimulated human dental pulp cells (HDPCs), compared to calcium hydroxide (Dycal®), a commonly used dental pulp capping material.
Materials and Methods: HDPCs cultured from three freshly extracted intact third molars were incubated with 10 ng/ml of IL-1β to induce MMP-2 activity. Concentrations of Thai propolis extract (0.25-1.50 mg/ml) were evaluated with PrestoBlue cytotoxic assay. ProRoot®MTA mixed with distilled water, ProRoot®MTA mixed with non-toxic Thai propolis extract, non-toxic Thai propolis extract, and DyCal® were used to treat IL-1β-stimulated HDPCs in vitro. After 24 h of incubation, culture supernatants were collected and evaluated for MMP-2 expression using gelatin zymography.
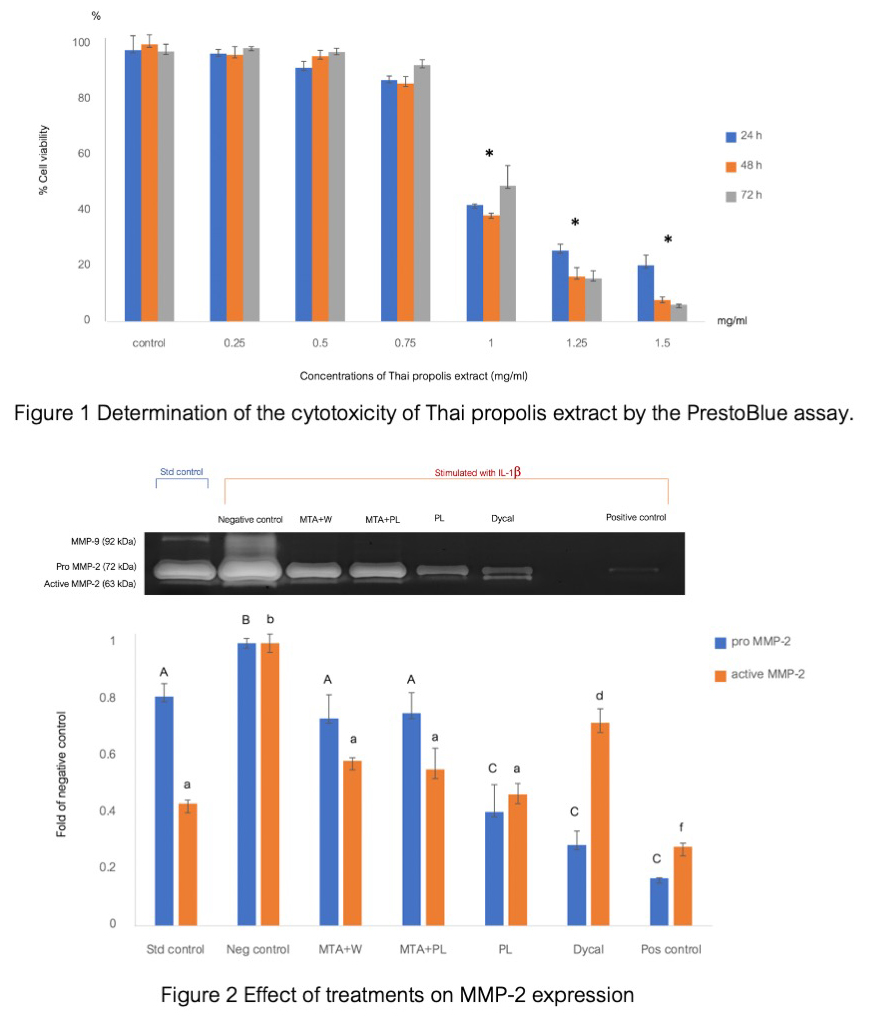
Results: Thai propolis extract 0.25, 0.50, and 0.75 mg/ml were not toxic to inflamed pulp cells at 24, 48, and 72 h. MMP-2 expression was upregulated in IL-1β–challenged HDPCs. Pro-form of MMP-2 was significantly reduced when IL-1β–stimulated HDPCs were incubated with 0.75 mg/ml Thai propolis extract and Dycal®, lower than MTA mixed with propolis and untreated control. In addition, Thai propolis extract significantly decreased the levels of active MMP-2 than Dycal®. However, the MMP-2 levels were not statistically different among MTA mixed with distilled water and MTA mixed with the propolis extract.
Conclusion: Thai propolis extract reduced an active form of MMP-2, greater than Dycal®. Although Thai propolis extract and Dycal® provide better MMP-2 reduction than MTA groups, Thai propolis extract mixed with MTA showed an insignificant effect on MMP-2 reduction in IL-1β – stimulated HDPCs.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Palosaari H, Pennington CJ, Larmas M, Edwards DR, Tjäderhane L, Salo T. Expression profile of matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs in mature human odontoblasts and pulp tissue. Eur J Oral Sci. 2003 Apr; 111(2):117–127. doi: 10.1034/j.1600-0722.2003.00026.x.
Mazzoni A, Mannello F, Tay FR, Tonti GA, Papa S, Mazzotti G, et al. Zymographic analysis and characterization of MMP-2 and -9 forms in human sound dentin. J Dent Res. 2007 May; 86(5):436–440. doi: 10.1177/154405910708600509.
Shin SJ, Lee JI, Baek SH, Lim SS. Tissue levels of matrix metalloproteinases in pulps and periapical lesions. J Endod. 2002 Apr; 28(4):313–315. doi: 10.1097/00004770-200204000-00013.
Chang YC, Yang SF, Hsieh YS. Regulation of matrix metalloproteinase-2 production by cytokines and pharmacological agents in human pulp cell cultures. J Endod. 2001 Nov; 27(11):679–682. doi: 10.1097/00004770-200111000-00007.
Huang FM, Yang SF, Hsieh YS, Liu CM, Yang LC, Chang YC. Examination of the signal transduction pathways involved in matrix metalloproteinases-2 in human pulp cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004 Mar; 97(3):398–403. doi: 10.1016/j.tripleo.2003.10.007.
Cho SY, Seo DG, Lee SJ, Lee J, Lee SJ, Jung IY. Prognostic factors for clinical outcomes according to time after direct pulp capping. J Endod. 2013 Mar; 39(3):327–331. doi: 10.1016/j.joen.2012.11.034.
Zuh C, Ju B, Ni R. Clinical outcome of direct pulp capping with MTA or calcium hydroxide: a systematic review and meta-analysis. Int J Clin Exp Med. 2015 Oct; 8(10):17055–17060.
Chen CL, Kao CT, Ding SJ, Shie MY, Huang TH. Expression of the inflammatory marker cyclooxygenase-2 in dental pulp cells cultured with mineral trioxide aggregate or calcium silicate cements. J Endod. 2010 Mar; 36(3):465–468. doi: 10.1016/j.joen.2009.12.008.
Reyes-Carmona JF, Santos AR, Figueiredo CP, Felippe MS, Felippe WT, Cordeiro MM. In vivo host interactions with mineral trioxide aggregate and calcium hydroxide: inflammatory molecular signaling assessment. J Endod. 2011 Sep; 37(9):1225–1235. doi: 10.1016/j.joen.2011.05.031.
Barczak K, Palczewska-Komsa M, Lipski M, Chlubek D, Buczkowska-Radlińska J, Baranowska-Bosiacka I. The influence of new silicate cement mineral trioxide aggregate (MTA Repair HP) on metalloproteinase MMP-2 and MMP-9 expression in cultured THP-1 macrophages. Int J Mol Sci. 2020 Dec; 22(1):295. doi: 10.3390/ijms22010295. PMID: 33396675.
Lee YY, Li YC, Hung SL, Chen YC, Lee YH, Yang SF. Mineral trioxide aggregate induces the release of matrix metalloproteinase-9 by human neutrophils. J Dent Sci. 2013 Dec; 8(4):378–384. doi: 10.1016/j.jds.2012.12.010.
S VK. Propolis in dentistry and oral cancer management. N Am J Med Sci. 2014 Jun; 6(6): 250-259. doi: 10.4103/1947-2714.134369.
Kantrong N, Kumtawee J, Damrongrungruang T, Puasiri S, Makeudom A, Krisanaprakornkit S, et al. An in vitro anti-inflammatory effect of Thai propolis in human dental pulp cells. J Appl Oral Sci. 2023 Jun; 31:e20230006. doi: 10.1590/1678-7757-2023-0006.
Likitpongpipat N, Sangmaneedet S, Klanrit P, Noisombut R, Krisanaprakornkit S, Chailertvanitkul P. Promotion of dental pulp wound healing in New Zealand white rabbits’ teeth by Thai propolis product. J Vet Dent. 2019 Mar; 36(1):17–24. doi: 10.1177/0898756418818891.
Prueksakorn A, Puasiri S, Ruangsri S, Makeudom A, Sastraruji T, Krisanaprakornkit S, et al. The preservative effect of Thai propolis extract on the viability of human periodontal ligament cells. Dent Traumatol. 2016 Dec; 32(6):495–501. doi: 10.1111/edt.12292.
Okamoto M, Takahashi Y, Komichi S, Cooper PR, Hayashi M. Dentinogenic effects of extracted dentin matrix components digested with matrix metalloproteinases. Sci Rep. 2018 Jul; 8(1):10690. doi: 10.1038/s41598-018-29112-3.
Shi B, Zhao Y, Yuan X. Effects of MTA and Brazilian propolis on the biological properties of dental pulp cells. Braz Oral Res. 2020 Jan; 33:e117. doi: 10.1590/1807-3107bor-2019.vol33.0117.
International Organiation for Standardization – ISO. ISO 10993-12:2012 Biological evaluation of medical devices. Part 12: Sample preparation and reference materials. Geneva: International Organiation for Standardization; 2012.
Gusman H, Santana RB, Zehnder M. Matrix metalloproteinase levels and gelatinolytic activity in clinically healthy and inflamed human dental pulps. Eur J Oral Sci. 2002 Oct; 110(5):353–357. doi: 10.1034/j.1600-0722.2002.21347.x.
Wisithphrom K, Windsor LJ. The effects of tumor necrosis factor-alpha, interleukin-1beta, interleukin-6, and transforming growth factor-beta1 on pulp fibroblast mediated collagen degradation. J Endod. 2006 Sep; 32(9):853–861. doi: 10.1016/j.joen.2006.03.017.
Sivula A, Talvensaari-Mattila A, Lundin J, Joensuu H, Haglund C, Ristimäki A, et al. Association of cyclooxygenase-2 and matrix metalloproteinase-2 expression in human breast cancer. Breast Cancer Res Treat. 2005 Feb; 89(3):215–220. doi: 10.1007/s10549-004-0714-4.
Kuratate M, Yoshiba K, Shigetani Y, Yoshiba N, Ohshima H, Okiji T. Immunohistochemical analysis of nestin, osteopontin, and proliferating cells in the reparative process of exposed dental pulp capped with mineral trioxide aggregate. J Endod. 2008 Aug;34(8):970-974. doi: 10.1016/j.joen.2008.03.021.
Mirzoeva OK, Calder PC. The effect of propolis and its components on eicosanoid production during the inflammatory response. Prostaglandins Leukot Essent Fatty Acids. 1996 Dec; 55(6):441–449. doi: 10.1016/s0952-3278(96)90129-5.
Kale Y, Yadav S, Dadpe M, Dahake P, Kendre S. Bioinductive and anti-inflammatory properties of Propolis and Biodentine on SHED. Saudi Dent J. 2022 Nov; 34(7):544–552. doi: 10.1016/j.sdentj.2022.08.009.
Wang Q, Sui X, Sui DJ, Yang P. Flavonoid extract from Propolis inhibits cardiac fibrosis triggered by myocardial infarction through upregulation of SIRT1. Evid Based Complement Alternat Med. 2018 Jun;2018:4957573. doi: 10.1155/2018/4957573.
Jin UH, Chung TW, Kang SK, Suh SJ, Kim JK, Chung KH, et al. Caffeic acid phenyl ester in propolis is a strong inhibitor of matrix metalloproteinase-9 and invasion inhibitor: Isolation and identification. Clinica Chimica Acta. 2005 Dec; 362(1-2):57-64. doi: 10.1016/j.cccn.2005.05.009.
Peng CY, Yang HW, Chu YH, Chang YC, Hsieh MJ, Chou MY, et al. Caffeic acid phenethyl ester inhibits oral cancer cell metastasis by regulating matrix metalloproteinase-2 and the mitogen-activated protein kinase pathway. Evid Based Complement Alternat Med. 2012; 2012:732578. doi: 10.1155/2012/732578.
Chaussain C, Boukpessi T, Khaddam M, Tjaderhane L, George A, Menashi S. Dentin matrix degradation by host matrix metalloproteinases: inhibition and clinical perspectives toward regeneration. Front Physiol. 2013 Nov;4:308. doi: 10.3389/fphys.2013.00308.
Kim JM, Kang SW, Shin SM, Su Kim D, Choi KK, Kim EC, et al. Inhibition of matrix metalloproteinases expression in human dental pulp cells by all-trans retinoic acid. Int J Oral Sci. 2014 Sep; 6(3):150–153. doi: 10.1038/ijos.2013.63.
Charadram N, Farahani RM, Harty D, Rathsam C, Swain MV, Hunter N. Regulation of reactionary dentin formation by odontoblasts in response to polymicrobial invasion of dentin matrix. Bone. 2012 Jan; 50(1):265–275. doi: 10.1016/j.bone.2011.10.031.
Gou X, Xue Y, Zheng H, Yang G, Chen S, Chen Z, et al. Gelatinases cleave dentin sialoprotein intracellularly. Front Physiol. 2020 Jun;11:686. doi: 10.3389/fphys.2020.00686.
Kim JH, Kim SY, Woo SM, Jeong HN, Jung JY, Kim SM, et al. Combination of mineral trioxide aggregate and propolis promotes odontoblastic differentiation of human dental pulp stem cells through ERK signaling pathway. Food Sci Biotechnol. 2019 May; 28(6):1801–1809. doi: 10.1007/s10068-019-00609-5.
Keiser K, Johnson CC, Tipton DA. Cytotoxicity of mineral trioxide aggregate using human periodontal ligament fibroblasts. J Endod. 2000 May; 26(5):288–291. doi: 10.1097/00004770-200005000-00010.
Snoek-van Beurden PA, Von den Hoff JW. Zymographic techniques for the analysis of matrix metalloproteinases and their inhibitors. Biotechniques. 2005 Jan; 38(1):73–83. doi: 10.2144/05381RV01.