Nicotiana benthamiana-derived epidermal growth factor rescues human oral keratinocytes exposed to 5-fluorouracil cytotoxicity
Main Article Content
Abstract
Objectives: 5-fluorouracil (5-FU) is commonly used as a chemotherapy drug for head and neck cancers. However, it often produces an adverse cytotoxic effect on the oral epithelia leading to oral mucositis (OM) in cancer patients. Thus, reversing this cytotoxic effect locally with novel bioactive drugs or factors is necessary. This study aimed to investigate the rescue effect of Nicotiana benthamiana plant-derived epidermal growth factor (P-EGF) on human normal oral keratinocytes (NOKs) exposed to 5-FU-induced cytotoxicity.
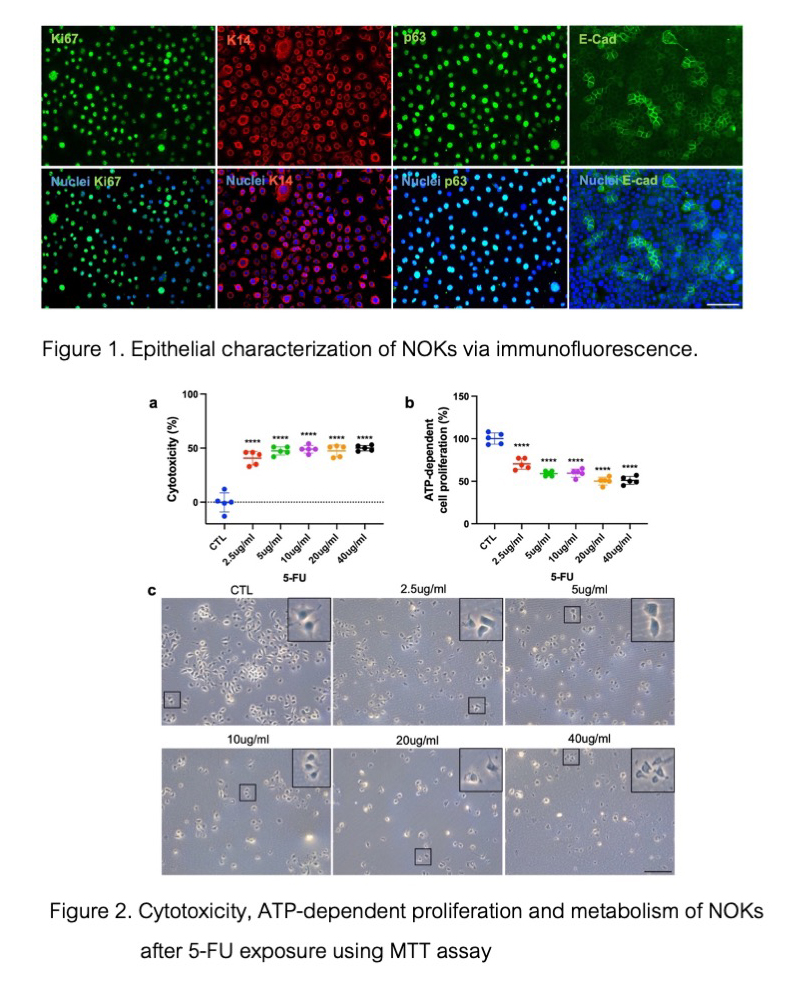
Materials and Methods: Cytotoxicity and ATP-dependent proliferation and metabolism of NOK monolayer cultures were assessed after 48 hours of 5-FU exposure and 48 hours of P-EGF treatment using MTT and luciferase-based ATP assays, respectively. Cell death was determined with Live/Dead staining using calcein-AM and propidium iodide. Cell recolonization was evaluated by in vitro scratch assay. Five biological replicates were run. One-way ANOVA with post hoc Dunnett’s test was used for statistical analysis with GraphPad Prism 9.5.1 software.
Results: P-EGF at 80ng/ml significantly promoted cell proliferation and metabolism after 5-FU-induced cytotoxicity (p-value=0.0006). Additionally, the recolonization of NOKs was also significantly enhanced by the addition of P-EGF (20, 40 and 80 ng/ml, p-value<0.0001).
Conclusion(s): P-EGF rescued the proliferation of 5-FU-exposed NOKs as well as induced epithelial cell recolonization. Thus, P-EGF could be a potential therapeutic approach for OM.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Berger K, Schopohl D, Bollig A, Strobach D, Rieger C, Rublee D, et al. Burden of oral mucositis: a systematic review and implications for future research. Oncol Res Treat. 2018 May;41(6):399-405. doi: 10.1159/000487085.
Sonis ST. The pathobiology of mucositis. Nat Rev Cancer. 2004 Apr;4(4):277-284. doi: 10.1038/nrc1318.
Elad S, Cheng KKF, Lalla RV, Yarom N, Hong C, Logan RM, et al. Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO). MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2020 Oct;126(19):4423-4431. doi: 10.1002/cncr.33100.
Elting LS, Chang YC. Costs of oral complications of cancer therapies: estimates and a blueprint for future study. J Natl Cancer Inst Monogr. 2019 Aug;2019 (53):lgz010. doi: 10.1093/jncimonographs/lgz010.
Riley P, Glenny AM, Worthington HV, Littlewood A, Mauleffinch LMF, Clarkson JE, et al. Interventions for preventing oral mucositis in patients with cancer receiving treatment: cytokines and growth factors. Cochrane Database Syst Rev. 2017 Nov;11(11):CD011990. doi: 10.1002/14651858.CD011990.pub2.
Coutsouvelis J, Corallo C, Spencer A, Avery S, Dooley M, Kirkpatrick CM. A meta-analysis of palifermin efficacy for the management of oral mucositis in patients with solid tumours and haematological malignancy. Crit Rev Oncol Hematol. 2022 Apr;172:103606. doi: 10.1016/j.critrevonc.2022.103606.
McDonnell AM, Lenz KL. Palifermin: role in the prevention of chemotherapy-and radiation-induced mucositis. Ann Pharmacother. 2007 Jan;41(1):86-94. doi: 10.1345/aph.1G473.
Chen J, Bekale LA, Khomtchouk KM, Xia A, Cao Z, Ning S, et al. Locally administered heparin-binding epidermal growth factor-like growth factor reduces radiation-induced oral mucositis in mice. Sci Rep. 2020 Oct;10(1):17327. doi: 10.1038/s41598-020-73875-7.
Wu HG, Song SY, Kim YS, Oh YT, Lee CG, Keum KC, et al. Therapeutic effect of recombinant human epidermal growth factor (RhEGF) on mucositis in patients undergoing radiotherapy, with or without chemotherapy, for head and neck cancer: a double‐blind placebo‐controlled prospective phase 2 multi‐institutional clinical trial. Cancer. 2009 Aug;115(16):3699-3708. doi: 10.1002/cncr.24414.
Kim KI, Kim JW, Lee HJ, Kim BS, Bang SM, Kim I, et al. Recombinant human epidermal growth factor on oral mucositis induced by intensive chemotherapy with stem cell transplantation. Am J Hematol. 2013 Feb;88(2):107-112. doi: 10.1002/ajh.23359.
Langlais C, Korn B. Recombinant protein expression in bacteria. In: Detlev G, Klaus R, editors. Encyclopedic reference of genomics and proteomics in molecular medicine. Springer Berlin Heidelberg, 2006; p. 1609-1116.
Shanmugaraj B, I. Bulaon CJ, Phoolcharoen W. Plant molecular farming: A viable platform for recombinant biopharmaceutical production. Plants (Basel). 2020 Jul;9(7):842. doi: 10.3390/plants9070842.
Hanittinan O, Oo Y, Chaotham C, Rattanapisit K, Shanmugaraj B, Phoolcharoen W. Expression optimization, purification and in vitro characterization of human epidermal growth factor produced in Nicotiana benthamiana. Biotechnol Rep (Amst). 2020 Sep;28:e00524. doi: 10.1016/j.btre.2020.e00524.
Phan TV, Oo Y, Rodboon T, Nguyen TT, Sariya L, Chaisuparat R, et al. Plant molecular farming-derived epidermal growth factor revolutionizes hydrogels for improving glandular epithelial organoid biofabrication. SLAS Technol. 2023 Aug;28(4):278-291. doi: 10.1016/j.slast.2023.03.002.
Hoshikawa E, Sato T, Haga K, Suzuki A, Kobayashi R, Tabeta K, et al. Cells/colony motion of oral keratinocytes determined by non-invasive and quantitative measurement using optical flow predicts epithelial regenerative capacity. Sci Rep. 2021 May;11(1):10403. doi: 10.1038/s41598-021-89073-y.
Bikle DD, Xie Z, Tu CL. Calcium regulation of keratinocyte differentiation. Expert Rev Endocrinol Metab. 2012 Jul;7(4):461-472. doi: 10.1586/eem.12.34.
Suarez-Arnedo A, Figueroa FT, Clavijo C, Arbeláez P, Cruz JC, Muñoz-Camargo C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PloS One. 2020 Jul;15(7):e0232565. doi: 10.1371/journal.pone.0232565.
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001 Feb;2(2):127-137. doi: 10.1038/35052073.
Dammen-Brower K, Epler P, Zhu S, Bernstein ZJ, Stabach PR, Braddock DT, et al. Strategies for glycoengineering therapeutic proteins. Front Chem. 2022 Apr;10:863118. doi: 10.3389/fchem.2022.863118.
Hunter M, Yuan P, Vavilala D, Fox M. Optimization of protein expression in mammalian cells. Curr Protoc Protein Sci. 2019 Feb;95(1):e77. doi: 10.1002/cpps.77.
Duval K, Grover H, Han LH, Mou Y, Pegoraro AF, Fredberg J, et al. Modeling physiological events in 2D vs. 3D cell culture. Physiology (Bethesda). 2017 Jul;32(4):266-277. doi: 10.1152/physiol.00036.2016.
Bai JY, Zeng L, Hu YL, Li YF, Lin ZP, Shang SC, et al. Expression and characteristic of synthetic human epidermal growth factor (hEGF) in transgenic tobacco plants. Biotechnol Lett. 2007 Dec;29:2007-2012. doi: 10.1007/s10529-007-9438-y.