Primary contraction of harvested palatal sub-epithelial connective tissue grafts: a pilot study
Main Article Content
Abstract
Objective(s): To elucidate and compare the primary contraction of palatal sub-epithelial connective tissue grafts after harvesting at different times.
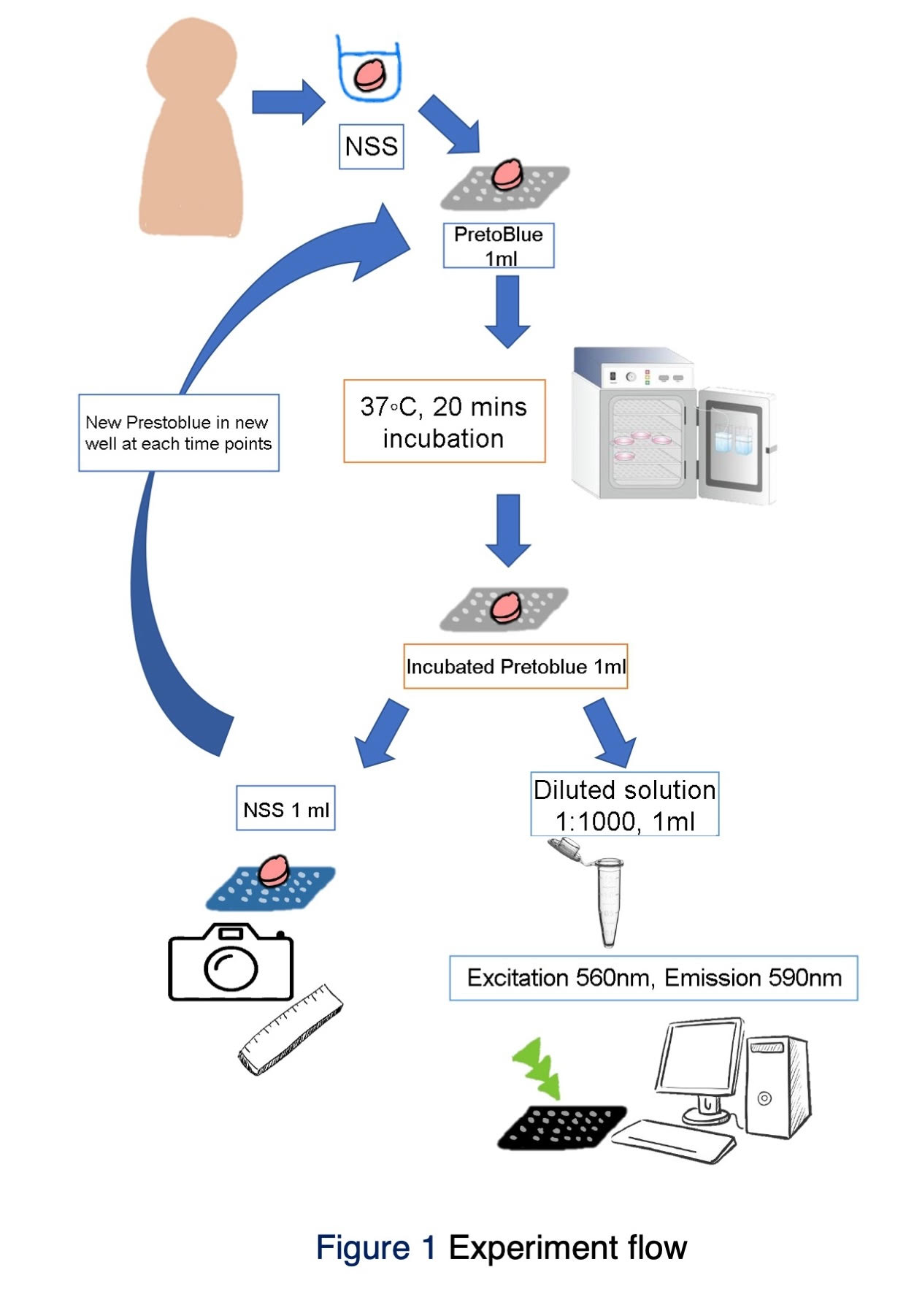
Materials and Methods: Ten patients who underwent soft tissue graft treatment, free gingival graft, or connective tissue graft. A 4-mm biopsy punch took each sample with 1 mm thickness after 1-mm-depth de-epithelialization. A total of 10 samples were recorded for graft contraction by a standardized photograph taken at 20(T1), 40(T2), 60(T3), 90(T4), 120 minutes(T5), and 24 hours(T6). Throughout the contraction investigation, the sample was incubated at 37 degrees Celsius, washed twice, and immersed in a new normal saline solution at 4 degrees Celsius between time points. The graft area was computed using ImageJ software. The graft contraction was calculated as the average percent of the original area (% of T1). Differences in graft contraction were analyzed using Friedman's Two-Way Analysis of Variance by Ranks and pairwise comparisons.
Results: Only one significant difference in graft contraction was found between T4 and T2, meaning that the graft area at 90 minutes decreased compared to the graft area at 40 minutes (p-value =0.047).
Conclusion: The harvested palatal sub-epithelial connective tissue graft size remained constant, except at 90 minutes after harvesting when it significantly decreased compared to 40 minutes. This study indicates that the sub-epithelial connective tissue graft can stay in normal saline solution outside the oral cavity with minimal contraction.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Klokkevold P, Newman MG, Takei H, Carranza F. Newman and Carranza's Clinical Periodontology. 2018
Thoma DS, Zeltner M, Hilbe M, Hämmerle CH, Hüsler J, Jung RE. Randomized controlled clinical study evaluating effectiveness and safety of a volume-stable collagen matrix compared to autogenous connective tissue grafts for soft tissue augmentation at implant sites. J Clin Periodontol. 2016 Oct;43(10):874-885. doi: 10.1111/jcpe.12588.
Chiu YW, Lee SY, Lin YC, Lai YL. Significance of the width of keratinized mucosa on peri-implant health. J Chin Med Assoc. 2015 Jul;78(7):389-394. doi: 10.1016/j.jcma.2015.05.001.
Sanz M, Lorenzo R, Aranda JJ, Martin C, Orsini M. Clinical evaluation of a new collagen matrix (Mucograft prototype) to enhance the width of keratinized tissue in patients with fixed prosthetic restorations: a randomized prospective clinical trial. J Clin Periodontol. 2009 Oct;36(10):868-876. doi: 10.1111/j.1600-051X.2009.01460.x.
Pini Prato G. Mucogingival deformities. Ann Periodontol. 1999 Dec;4(1):98-101. doi: 10.1902/annals.1999.4.1.98.
Cortellini P, Bissada NF. Mucogingival conditions in the natural dentition: Narrative review, case definitions, and diagnostic considerations. J Periodontol. 2018 Jun;89 Suppl 1:S204-S213. doi: 10.1002/JPER.16-0671.
Pini Prato G, Di Gianfilippo R. On the value of the 2017 classification of phenotype and gingival recessions. J Periodontol. 2021 May; 92(5): 613–618. https://doi.org/10.1002/JPER.20-0487
Hosanguan C, Ungchusak C, Leelasithorn S, Prasertsom P. The extent and correlates of gingival recession in non-institutionalised Thai elderly. J Int Acad Periodontol. 2002 Oct;4(4):143-148.
Gorman WJ. Prevalence and etiology of gingival recession. J Periodontol. 1967 Jul-Aug;38(4):316-322. doi: 10.1902/jop.1967.38.4.316.
Slutzkey S, Levin L. Gingival recession in young adults: occurrence, severity, and relationship to past orthodontic treatment and oral piercing. Am J Orthod Dentofacial Orthop. 2008 Nov;134(5):652-656. doi: 10.1016/j.ajodo.2007.02.054.
Chambrone L, Pannuti CM, Tu YK, Chambrone LA. Evidence-based periodontal plastic surgery. II. An individual data meta-analysis for evaluating factors in achieving complete root coverage. J Periodontol. 2012 Apr;83(4):477-490. doi: 10.1902/jop.2011.110382.
Thoma DS, Benić GI, Zwahlen M, Hämmerle CH, Jung RE. A systematic review assessing soft tissue augmentation techniques. Clin Oral Implants Res. 2009 Sep; 20 Suppl 4:146-165. doi: 10.1111/j.1600-0501.2009.01784.x.
Langer B, Langer L. Subepithelial connective tissue graft technique for root coverage. J Periodontol. 1985 Dec;56(12):715-720. doi: 10.1902/jop.1985.56.12.715.
Thoma DS, Buranawat B, Hämmerle CH, Held U, Jung RE. Efficacy of soft tissue augmentation around dental implants and in partially edentulous areas: a systematic review. J Clin Periodontol. 2014 Apr;41 Suppl 15:S77-S91. doi: 10.1111/jcpe.12220.
Edel A. Clinical evaluation of free connective tissue grafts used to increase the width of keratinised gingiva. J Clin Periodontol. 1974;1(4):185-196. doi: 10.1111/j.1600-051x.1974.tb01257.x.
Wessel JR, Tatakis DN. Patient outcomes following subepithelial connective tissue graft and free gingival graft procedures. J Periodontol. 2008 Mar;79(3):425-430. doi: 10.1902/jop.2008.070325.
Yotnuengnit P, Laohspand P, Kerdvongbundit T, Yosvimol K. Gingival tissue graft: practicable techniques. Department of Oral Medicine and Periodontology, Faculty of Dentistry, Mahidol University; 2014.
Sullivan HC, Atkins JH. Free autogenous gingival grafts. I. Principles of successful grafting. Periodontics. 1968 Jun;6(3):121-129.
Boncler M, Różalski M, Krajewska U, Podsędek A, Watala C. Comparison of PrestoBlue and MTT assays of cellular viability in the assessment of anti-proliferative effects of plant extracts on human endothelial cells. J Pharmacol Toxicol Methods. 2014 Jan-Feb;69(1):9-16. doi: 10.1016/j.vascn.2013.09.003.
Yu SK, Lee MH, Park BS, Jeon YH, Chung YY, Kim HJ. Topographical relationship of the greater palatine artery and the palatal spine. Significance for periodontal surgery. J Clin Periodontol. 2014 Sep;41(9):908-913. doi: 10.1111/jcpe.12288.
Kim DH, Won SY, Bae JH, Jung UW, Park DS, Kim HJ, et al. Topography of the greater palatine artery and the palatal vault for various types of periodontal plastic surgery. Clin Anat. 2014 May;27(4):578-584. doi: 10.1002/ca.22252.
Fu JH, Hasso DG, Yeh CY, Leong DJ, Chan HL, Wang HL. The accuracy of identifying the greater palatine neurovascular bundle: a cadaver study. J Periodontol. 2011 Jul;82(7):1000-1006. doi: 10.1902/jop.2011.100619.
Benninger B, Andrews K, Carter W. Clinical measurements of hard palate and implications for subepithelial connective tissue grafts with suggestions for palatal nomenclature. J Oral Maxillofac Surg. 2012 Jan;70(1):149-153. doi: 10.1016/j.joms.2011.03.066.
Orsini M, Orsini G, Benlloch D, Aranda JJ, Lázaro P, Sanz M. Esthetic and dimensional evaluation of free connective tissue grafts in prosthetically treated patients: a 1-year clinical study. J Periodontol. 2004 Mar;75(3):470-477. doi: 10.1902/jop.2004.75.3.470.
Hämmerle CH, Giannobile WV; Working Group 1 of the European Workshop on Periodontology. Biology of soft tissue wound healing and regeneration--consensus report of Group 1 of the 10th European Workshop on Periodontology. J Clin Periodontol. 2014 Apr;41 Suppl 15:S1-S5. doi: 10.1111/jcpe.12221.
Del Pizzo M, Modica F, Bethaz N, Priotto P, Romagnoli R. The connective tissue graft: a comparative clinical evaluation of wound healing at the palatal donor site. A preliminary study. J Clin Periodontol. 2002 Sep;29(9):848-854. doi: 10.1034/j.1600-051x.2002.290910.x.
Khaw PT, Occleston NL, Schultz G, Grierson I, Sherwood MB, Larkin G. Activation and suppression of fibroblast function. Eye (Lond). 1994;8 (Pt 2):188-195. doi: 10.1038/eye.1994.44.
McLeod DE, Reyes E, Branch-Mays G. Treatment of multiple areas of gingival recession using a simple harvesting technique for autogenous connective tissue graft. J Periodontol. 2009 Oct;80(10):1680-1687. doi: 10.1902/jop.2009.090187.
Blumberg N, Cholette JM, Pietropaoli AP, Phipps R, Spinelli SL, Eaton MP, et al. 0.9% NaCl (Normal Saline) - Perhaps not so normal after all? Transfus Apher Sci. 2018 Feb;57(1):127-131. doi: 10.1016/j.transci.2018.02.021.
Said KN, Abu Khalid AS, Farook FF. Anatomic factors influencing dimensions of soft tissue graft from the hard palate. A clinical study. Clin Exp Dent Res. 2020 Aug;6(4):462-469. doi: 10.1002/cre2.298.
Sultana T, Dayem AA, Lee SB, Cho SG, Lee JI. Effects of carrier solutions on the viability and efficacy of canine adipose-derived mesenchymal stem cells. BMC Vet Res. 2022 Jan 7;18(1):26. doi: 10.1186/s12917-021-03120-4.
Lall N, Henley-Smith CJ, De Canha MN, Oosthuizen CB, Berrington D. Viability reagent, PrestoBlue, in comparison with other available reagents, utilized in cytotoxicity and antimicrobial assays. Int J Microbiol. 2013;2013:420601. doi: 10.1155/2013/420601.
Xu M, McCanna DJ, Sivak JG. Use of the viability reagent PrestoBlue in comparison with alamarBlue and MTT to assess the viability of human corneal epithelial cells. J Pharmacol Toxicol Methods. 2015 Jan-Feb;71:1-7. doi: 10.1016/j.vascn.2014.11.003.