A decellularized extracellular matrix hydrogel to promote the proliferation of human salivary gland cells
Main Article Content
Abstract
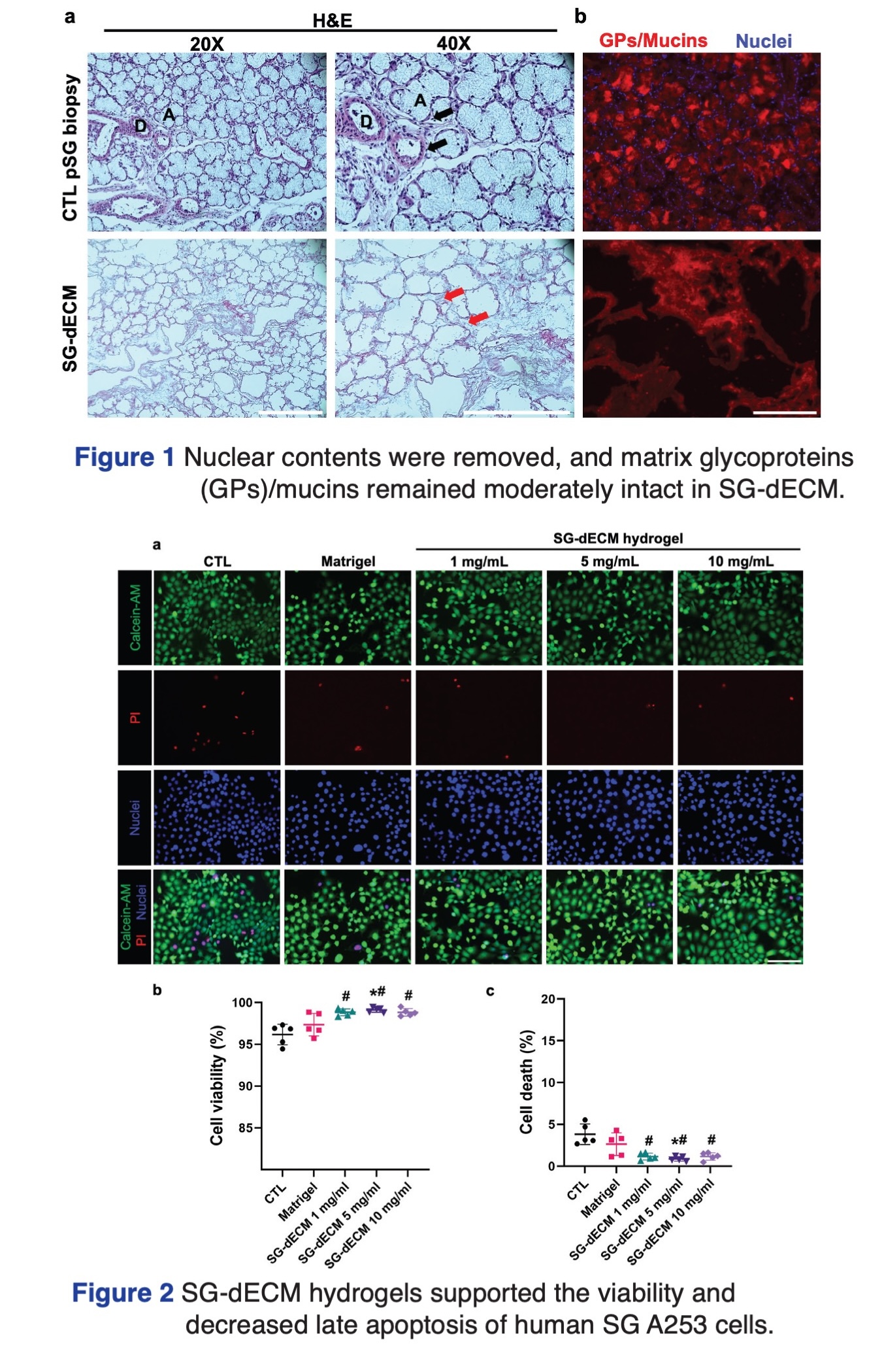
Objectives: Matrigel, a raw matrix from the Engelbreth-Holm-Swarm mouse sarcoma, has been commonly used to promote salivary gland (SG) cell assembly and proliferation in vitro, however, it possesses limitations such as batch-to-batch variations and undefined tumor-derived components, hence, alternative matrices are lacking. This study aimed to develop porcine submandibular gland decellularized extracellular matrix (SG-dECM) hydrogels to support SG cell viability and proliferation.
Materials and methods: SG-dECM was produced using non-ionic and ionic detergent perfusions, then digested with a pepsin-based HCl buffer to generate SG-dECM hydrogels at concentrations of 1, 5, and 10 mg/mL. SG-dECM sections were stained with hematoxylin and eosin (H&E), and rhodamine-labeled peanut agglutinin staining of glycoproteins/mucins. A human submandibular gland cell line, A253 (HTB-41TM, ATCC), was cultured as a monolayer culture with SG-dECM hydrogel- or Matrigel-coated on 96-well plates and assessed for proliferation over 4 days using an ATP-dependent assay. Uncoated wells were used as negative controls. Viable and late apoptotic cells were quantified with calcein-AM and propidium iodide staining, respectively. One- and two-way ANOVA with Tukey’s post-hoc tests were performed with 5 biological replicates.
Results: The developed SG-dECM retained moderately glycoproteins and mucins while cellular components were effectively removed. SG-dECM hydrogels at 5 mg/mL significantly enhanced A253 cell proliferation and viability compared to Matrigel (p<0.05) and uncoated wells (p<0.001) after 4 culture days.
Conclusion: SG-dECM hydrogels at 5 mg/mL promoted the proliferation and viability of A253 cells over 4 culture days. Thus, SG-dECM hydrogel could be a viable alternative to Matrigel for future drug screening applications.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Pedersen AM, Bardow A, Jensen SB, Nauntofte B. Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion. Oral Dis. 2002;8(3):117-29. doi: 10.1034/j.1601-0825.2002.02851.x.
Miranda-Rius J, Brunet-Llobet L, Lahor-Soler E, Farre M. Salivary Secretory Disorders, Inducing Drugs, and Clinical Management. Int J Med Sci. 2015;12(10):811-24. doi: 10.7150/ijms.12912.
Riley P, Glenny AM, Hua F, Worthington HV. Pharmacological interventions for preventing dry mouth and salivary gland dysfunction following radiotherapy. Cochrane Database Syst Rev. 2017;7(7):CD012744. doi: 10.1002/14651858.CD012744.
Kapalczynska M, Kolenda T, Przybyla W, Zajaczkowska M, Teresiak A, Filas V, et al. 2D and 3D cell cultures - a comparison of different types of cancer cell cultures. Arch Med Sci. 2018;14(4):910-9. doi: 10.5114/aoms.2016.63743.
Sui Y, Zhang S, Li Y, Zhang X, Hu W, Feng Y, et al. Generation of functional salivary gland tissue from human submandibular gland stem/progenitor cells. Stem Cell Res Ther. 2020;11(1):127. doi: 10.1186/s13287-020-01628-4.
Rebustini IT, Hoffman MP. ECM and FGF-dependent assay of embryonic SMG epithelial morphogenesis: investigating growth factor/matrix regulation of gene expression during submandibular gland development. Methods Mol Biol. 2009;522:319-30. doi: 10.1007/978-1-59745-413-1_21.
Kaur S, Kaur I, Rawal P, Tripathi DM, Vasudevan A. Non-matrigel scaffolds for organoid cultures. Cancer Lett. 2021;504:58-66. doi: 10.1016/j.canlet.2021.01.025.
Zhang X, Chen X, Hong H, Hu R, Liu J, Liu C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact Mater. 2022;10:15-31. doi: 10.1016/j.bioactmat.2021.09.014.
Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27(19):3675-83. doi: 10.1016/j.biomaterials.2006.02.014.
Gao Z, Wu T, Xu J, Liu G, Xie Y, Zhang C, et al. Generation of Bioartificial Salivary Gland Using Whole-Organ Decellularized Bioscaffold. Cells Tissues Organs. 2014;200(3-4):171-80. doi: 10.1159/000371873.
Shin K, Koo KH, Jeong J, Park SJ, Choi DJ, Ko YG, et al. Three-Dimensional Culture of Salivary Gland Stem Cell in Orthotropic Decellularized Extracellular Matrix Hydrogels. Tissue Eng Part A. 2019;25(19-20):1396-403. doi: 10.1089/ten.TEA.2018.0308.
Wang T, Huang Q, Rao Z, Liu F, Su X, Zhai X, et al. Injectable decellularized extracellular matrix hydrogel promotes salivary gland regeneration via endogenous stem cell recruitment and suppression of fibrogenesis. Acta Biomater. 2023;169:256-72. doi: 10.1016/j.actbio.2023.08.003.
Ahmed K, Rodboon T, Oo Y, Phan T, Chaisuparat R, Yodmuang S, et al. Biofabrication, biochemical profiling, and in vitro applications of salivary gland decellularized matrices via magnetic bioassembly platforms. Cell Tissue Res. 2023;392(2):499-516. doi: 10.1007/s00441-022-03728-4.
Liu C, Pei M, Li Q, Zhang Y. Decellularized extracellular matrix mediates tissue construction and regeneration. Front Med. 2022;16(1):56-82. doi: 10.1007/s11684-021-0900-3.
Freytes DO, Martin J, Velankar SS, Lee AS, Badylak SF. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials. 2008;29(11):1630-7. doi: 10.1016/j.biomaterials.2007.12.014.
Brightman AO, Rajwa BP, Sturgis JE, McCallister ME, Robinson JP, Voytik-Harbin SL. Time-lapse confocal reflection microscopy of collagen fibrillogenesis and extracellular matrix assembly in vitro. Biopolymers. 2000;54(3):222-34. doi: 10.1002/1097-0282(200009)54:3<222::AID-BIP80>3.0.CO;2-K.
Hoffman MP, Kibbey MC, Letterio JJ, Kleinman HK. Role of laminin-1 and TGF-beta 3 in acinar differentiation of a human submandibular gland cell line (HSG). J Cell Sci. 1996;109 ( Pt 8):2013-21. doi: 10.1242/jcs.109.8.2013.
Lam K, Zhang L, Bewick M, Lafrenie RM. HSG cells differentiated by culture on extracellular matrix involves induction of S-adenosylmethione decarboxylase and ornithine decarboxylase. J Cell Physiol. 2005;203(2):353-61. doi: 10.1002/jcp.20247.
Huang C, Sanaei F, Verdurmen WPR, Yang F, Ji W, Walboomers XF. The Application of Organs-on-a-Chip in Dental, Oral, and Craniofacial Research. J Dent Res. 2023;102(4):364-75. doi: 10.1177/00220345221145555.