The effect of different concentrations of radiopacifier agents on the radiopacity level of nano-calcium hydroxide intracanal medication
Main Article Content
Abstract
Objective: To assess radiopacity among nano-calcium hydroxide without and with the addition of various concentrations of barium sulfate as a radiopacifier.
Materials and Methods: The radiopacity of six materials: nano-calcium hydroxide, nano-calcium hydroxide with 25%, 30%, 35%, and 40% by weight of barium sulfate, and Ultracal XS® were evaluated according to ISO 6876:2012, with 5 specimens per group. Nano-calcium hydroxide and the various percentages by weight of barium sulfate were combined using a blending machine, then mixed with distilled water with a ratio of 1 g per ml. All samples were placed in the acrylic mold which was positioned alongside 0.5-mm increment aluminium step wedge and irradiated with x-ray 60 kV, 8 mA, for 0.2 seconds on a digital receptor. The radiopacity
of the materials was compared with the aluminium step wedge using the ImageJ program and transformed into mm of Al.
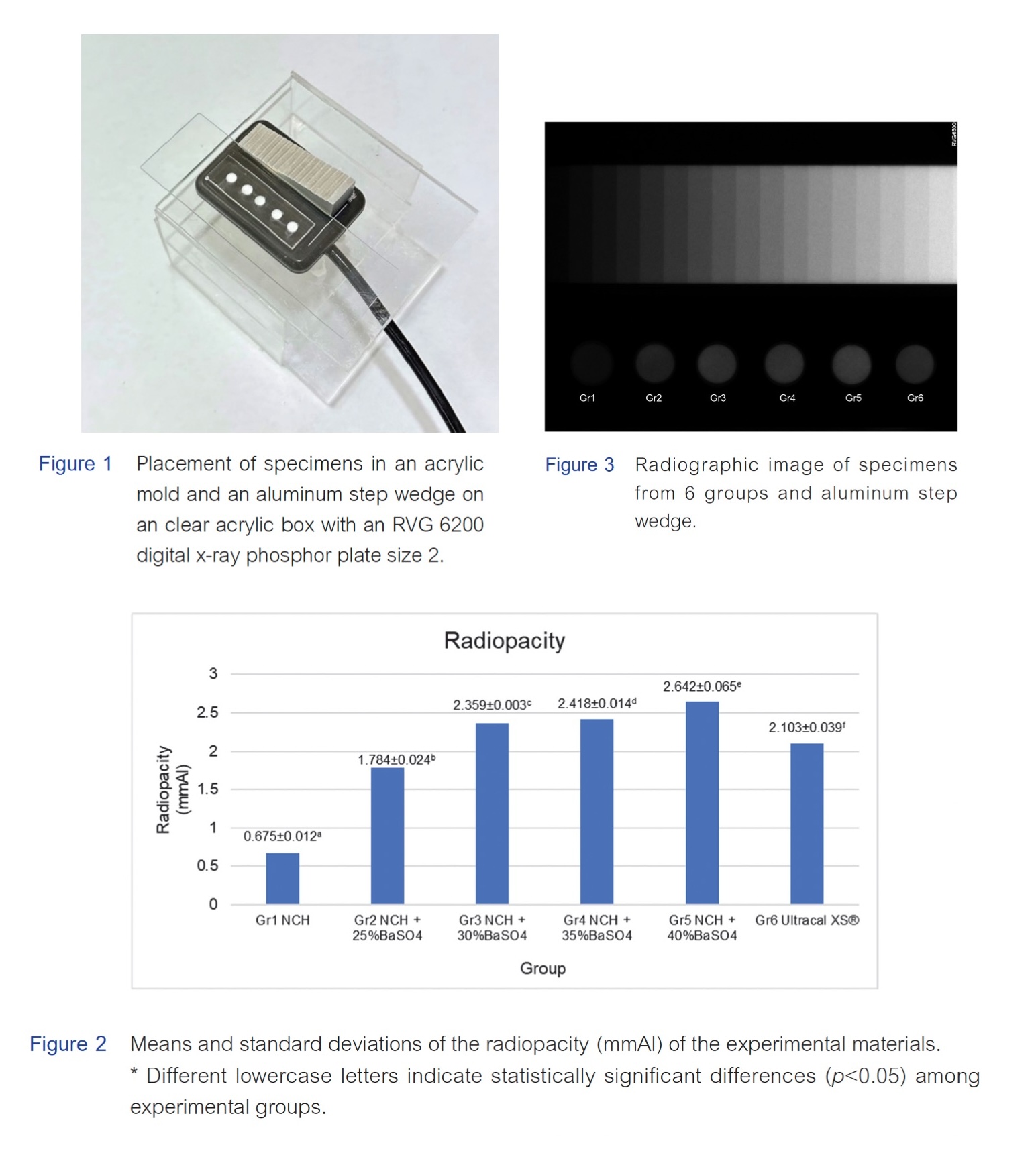
Results: The radiopacity of nano-calcium hydroxide ranged from 0.675 to 2.642 mmAl. The group without a radiopacifier exhibited the lowest radiopacity, followed by the groups with 25%, 30%, and 35% by weight of barium sulfate, while the highest radiopacity was observed in the group with 40% by weight of barium sulfate. Increasing the concentration of barium sulfate in nano-calcium hydroxide resulted in a proportional increase in radiopacity. Ultracal XS® showed lower radiopacity compared to nano-calcium hydroxide with 30% by weight of barium sulfate. Statistically significant differences in radiopacity were observed among all examined materials.
Conclusion: The radiopacity of nano-calcium hydroxide increases with the addition of barium sulfate. Approximately 30% by weight of BaSO4 should be added to achieve a radiopacity greater than 2 mmAl, based on an adaptation of the recommendation from the American National Standards Institute and the American Dental Association.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Siqueira Jr JF, Lopes HP. Mechanisms of antimicrobial activity of calcium hydroxide: a critical review. Int Endod J. 1999 Sep;32(5):361-369. doi: 10.1046/j.1365-2591.1999.00275.x.
Bystrom A, Sundqvist G. Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Eur J Oral Sci. 1981 Aug;89(4):321-328. doi: 10.1111/j.1600-0722.1981.tb01689.x.
Mohammadi Z, Dummer PMH. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int Endod J. 2011 Aug;44(8):697-730. doi: 10.1111/j.1365-2591.2011.01886.x.
Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced osteoclast-like cell functions on nanophase ceramics. Biomaterials. 2001 Jun;22(11):1327-1333. doi: 10.1016/s0142-9612(00)00285-4.
Dianat O, Saedi S, Kazem M, Alam M. Antimicrobial activity of nanoparticle calcium hydroxide against Enterococcus Faecalis: An in vitro study. Iran Endod J. 2015 Winter;10(1):39-43.
Zand V, Mokhtari H, Hasani A, Jabbari G. Comparison of the penetration depth of conventional and nano-particle calcium hydroxide into dentinal tubules. Iran Endod J. 2017 Summer;12(3):366-370. doi: 10.22037/iej.v12i3.16421.
Fava LR, Saunders WP. Calcium hydroxide pastes: classification and clinical indications. Int Endod J. 1999 Aug;32(4):257-282. doi: 10.1046/j.1365-2591.1999.00232.x.
Alaçam T, Görgül G, Omürlü H. Evaluation of diagnostic radiopaque contrast materials used with calcium hydroxide. J Endod. 1990 Aug;16(8):365-368. doi: 10.1016/S0099-2399(06)81907-2.
Barium sulfate. In: Aronson JK, editor. Meyler's Side Effects of Drugs (Sixteenth Edition). Oxford: Elsevier; 2016. p. 827-9.
Williams JA, Billington RW. A new technique for measuring the radiopacity of natural tooth substance and restorative materials. J Oral Rehabil. 1987 May;14(3):267-269. doi: 10.1111/j.1365-2842.1987.tb00718.x.
Hosney S, Abouelseoud HK, El-Mowafy O. Radiopacity of resin cements using digital radiography. J Esthet Restor Dent. 2017 May 6;29(3):215-221. doi: 10.1111/jerd.12288.
Ordinola-Zapata R, Bramante C, Garcia-Godoy F, Moldauer B, Minotti P, Grizzo L, et al. The effect of radiopacifiers agents on pH, calcium release, radiopacity, and antimicrobial properties of different calcium hydroxide dressings. Microsc Res Tech. 2015 Jul;78(7):620-625. doi: 10.1002/jemt.22521.
Prasetyo EP, Juniarti DE, Sampoerno G, Wahjuningrum DA, Budi AT, Hasri D, et al. The antibacterial efficacy of calcium hydroxide–iodophors and calcium hydroxide–barium sulfate root canal dressings on Enterococcus faecalis and Porphyromonas gingivalis in vitro. Dent. J. 2022 Jun;55(2):62-66. doi: 10.20473/j.djmkg.v55.i2.p62-66.
Caicedo R, Mercante D, Alongi D. Calcium-ion diffusion of four calcium hydroxide-based materials : Ultracal XS, Vitapex, Roeko calcium hydroxide plus points, and pure calcium hydroxide through radicular dentin. Int J Oral Med Scie. 2004 Jan;3:75-82. doi: 10.5466/ijoms.3.75.
Pibultip P, Piyachon C. Efficacy of Three irrigation protocols for removing three types of calcium hydroxide from root canals. Khon Kaen Dent J. 2022 Nov;25(3):1-11.
Nadar A, Muliya VS, Pai S, Pentapati KC. A comparative evaluation of calcium ion release and pH change using calcium hydroxide nanoparticles as intracanal medicament with different vehicles - An in vitro study. J Conserv Dent. 2023 Jan-Feb;26(1):47-51. doi: 10.4103/jcd.jcd_387_22.
Smith GN, Woods S. Organic iodine: a substitute for BaSO4 in apexification procedures. J Endod. 1983 Apr;9(4):153-155. doi: 10.1016/S0099-2399(83)80037-5.
Orucoglu H, Kont Cobankara F. Effect of unintentionally extruded calcium hydroxide plaste Including barium sulfate as a radiopaquing agent in treatment of teeth with periapical lesions: Report of a case. J Endod. 2008 Jul;34(7):888-891. doi: 10.1016/j.joen.2008.04.012.
Webber RT, Schwiebert KA, Cathey GM. A technique for placement of calcium hydroxide in the root canal system. J Am Dent Assoc. 1981 Sep;103(3):417-421. doi: 10.14219/jada.archive.1981.0346.
Anas A, Asaad J, Tarboush K. A Comparison of intra-oral digital imaging modalities: charged couple device versus storage phosphor plate. Int J Health Sci (Qassim). 2010 Nov;4(2):156-167.
Vibulcharoenkitja P, Banomyong D, Sutimuntanakul S. Radiopacity of biodentine, Bio-MA, and calcium silicate-based cement added with different radiopacifiers. M Dent J. 2019 Sep-Dec;39(3):199-205.
Wahidi A, Ha WN, Alvino L, Nagendrababu V, Rossi-Fedele G. Association between intracanal medicament radiopacity and streak artifact production using cone-beam computed tomography: A laboratory study. J Endod. 2023 Jul;49(7):909-914. doi: 10.1016/j.joen.2023.04.011.
Pekkan G. Radiopacity of dental materials: An Overview. Avicenna J Dent Res. 2016 Jun;8(2):8. doi: 10.17795/ajdr-36847.