Pyronaridine – the Current Antimalarial Standing Up to Parasite Resistance
DOI:
https://doi.org/10.33165/rmj.2024.47.4.270565Keywords:
Antimalarial resistancee, Artemisinin combination therapy, β-Hematin, Heme detoxification, Pynacrine, PyronaridineAbstract
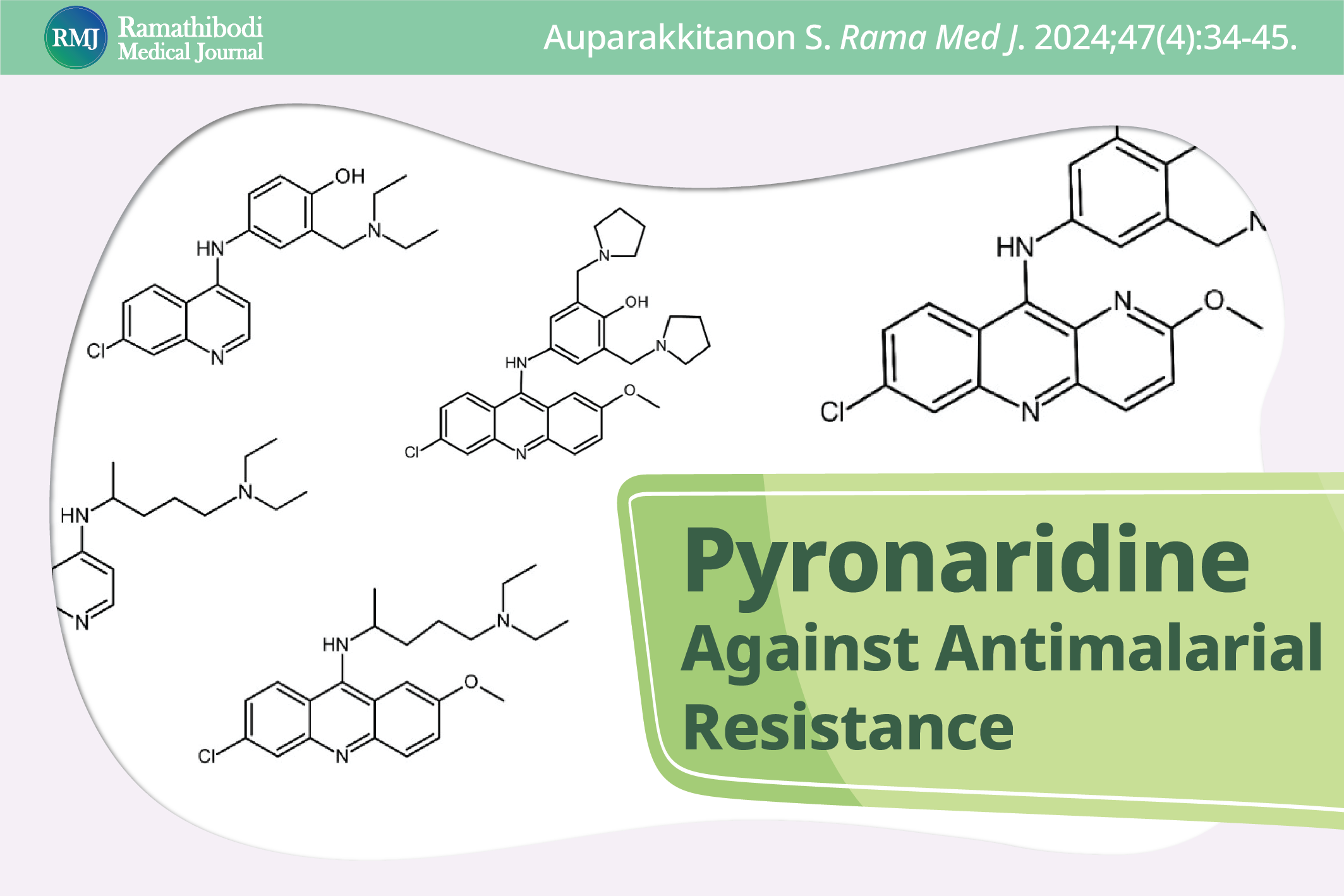
Pyronaridine, an aza-9-anilinoacridine schizonticide, was synthesized in China 54 years ago and since 2012 has been prescribed globally in combination with artesunate, marketed under the name Pyramax. Artesunate is an analog of artemisinin, a sesquiterpene lactone with an endoperoxide moiety, extracted from Artemisia annua L., and used as an herbal remedy in Chinese traditional medicine to treat jungle fever, also discovered in China at nearly the same time. Pyramax is one of the safest and most efficacious forms of artemisinin combination therapy (ACT) for treating uncomplicated Plasmodium falciparum malaria in adults and children. This narrative review explains the mechanism of action of pyronaridine, why it remains effective against P. falciparum even though the parasite has evolved resistance or tolerance to all other antimalarial drugs used in clinical practice, and suggests possible antiplasmodial deaza-pyronaridine (acridine) analogs that could be used should pyronaridine becomes ineffective.
References
World Health Organization. World malaria report 2023. World Health Organization. November 30, 2023. Access August 8, 2024. https://www.who.int/publications/i/item/9789240086173
Duffey M, Shafer RW, Timm J, et al. Combating antimicrobial resistance in malaria, HIV and tuberculosis. Nat Rev Drug Discov. 2024;23(6):461-479. doi:10.1038/s41573-024-00933-4
Zheng XY, Chen C, Gao FH, Zhu PE, Guo HZ. Synthesis of new antimalarial drug pyronaridine and its analogues. Yao Xue Xue Bao. 1982;17(2):118-125.
Tu Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat Med. 2011;17(10):1217-1220. doi:10.1038/nm.2471
Antinori S, Galimberti L, Milazzo L, Corbellino M. Plasmodium knowlesi: the emerging zoonotic malaria parasite. Acta Trop. 2013;125(2):191-201. doi:10.1016/j.actatropica.2012.10.008
Ashley EA, White NJ. Artemisinin-based combinations. Curr Opin Infect Dis. 2005;18(6):531-536. doi:10.1097/01.qco.0000186848.46417.6c
Croft SL, Duparc S, Arbe-Barnes SJ, et al. Review of pyronaridine anti-malarial properties and product characteristics. Malar J. 2012;11:270. doi:10.1186/1475-2875-11-270
Chu WY, Dorlo TPC. Pyronaridine: a review of its clinical pharmacology in the treatment of malaria. J Antimicrob Chemother. 2023;78(10):2406-2418. doi:10.1093/jac/dkad260
Pryce J, Taylor M, Fox T, Hine P. Pyronaridine-artesunate for treating uncomplicated Plasmodium falciparum malaria. Cochrane Database Syst Rev. 2022;6(6):CD006404. doi:10.1002/14651858.CD006404.pub4
Falade CO, Orimadegun AE, Olusola FI, et al. Efficacy and safety of pyronaridine-artesunate versus artemether-lumefantrine in the treatment of acute uncomplicated malaria in children in South-West Nigeria: an open-labelled randomized controlled trial. Malar J. 2023;22(1):154. doi:10.1186/s12936-023-04574-7
Shao BR. A review of antimalarial drug pyronaridine. Chin Med J (Engl). 1990;103(5):428-434.
Chavalitshewinkoon-Petmitr P, Pongvilairat G, Auparakkitanon S, Wilairat P. Gametocytocidal activity of pyronaridine and DNA topoisomerase II inhibitors against multidrug-resistant Plasmodium falciparum in vitro. Parasitol Int. 2000;48(4):275-280. doi:10.1016/s1383-5769(99)00028-8
Joice R, Nilsson SK, Montgomery J, et al. Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci Transl Med. 2014;6(244):244re5. doi:10.1126/scitranslmed.3008882
Stanisic DI, Good MF. Malaria vaccines: progress to date. BioDrugs. 2023;37(6):737-756. doi:10.1007/s40259-023-00623-4
Genton B. R21/Matrix-M™ malaria vaccine: a new tool to achieve WHO’s goal to eliminate malaria in 30 countries by 2030? J Travel Med. 2023;30(8):taad140. doi:10.1093/jtm/taad140
Dicko A, Ouedraogo JB, Zongo I, et al. Seasonal vaccination with RTS,S/AS01E vaccine with or without seasonal malaria chemoprevention in children up to the age of 5 years in Burkina Faso and Mali: a double-blind, randomised, controlled, phase 3 trial. Lancet Infect Dis. 2024;24(1):75-86. doi:10.1016/S1473-3099(23)00368-7
Wu LJ, Rabbege JR, Nagasawa H, Jacobs G, Aikawa M. Morphological effects of pyronaridine on malarial parasites. Am J Trop Med Hyg. 1988;38(1):30-36. doi:10.4269/ajtmh.1988.38.30
Auparakkitanon S, Chapoomram S, Kuaha K, Chirachariyavej T, Wilairat P. Targeting of hematin by the antimalarial pyronaridine. Antimicrob Agents Chemother. 2006;50(6):2197-2200. doi:10.1128/AAC.00119-06
Bailly C. Pyronaridine: an update of its pharmacological activities and mechanisms of action. Biopolymers. 2021;112(4):e23398. doi:10.1002/bip.23398
Auparakkitanon S, Wilairat P. Cleavage of DNA induced by 9-anilinoacridine inhibitors of topoisomerase II in the malaria parasite Plasmodium falciparum. Biochem Biophys Res Commun. 2000;269(2):406-409. doi:10.1006/bbrc.2000.2305
Ketron AC, Denny WA, Graves DE, Osheroff N. Amsacrine as a topoisomerase II poison: importance of drug-DNA interactions. Biochemistry. 2012;51(8):1730-1739. doi:10.1021/bi201159b
Francis SE, Sullivan DJ Jr, Goldberg DE. Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu Rev Microbiol. 1997;51:97-123. doi:10.1146/annurev.micro.51.1.97
Egan TJ. Recent advances in understanding the mechanism of hemozoin (malaria pigment) formation. J Inorg Biochem. 2008;102(5-6):1288-1299. doi:10.1016/j.jinorgbio.2007.12.004
Sullivan DJ Jr. Quinolines block every step of malaria heme crystal growth. Proc Natl Acad Sci USA. 2017;114(29):7483-7485. doi:10.1073/pnas.1708153114
Olafson KN, Nguyen TQ, Rimer JD, Vekilov PG. Antimalarials inhibit hematin crystallization by unique drug-surface site interactions. Proc Natl Acad Sci USA. 2017;114(29):7531-7536. doi:10.1073/pnas.1700125114
Auparakkitanon S, Wilairat P, Wilairat P. Will the in situ activator(s) of artemisinin please stand up? Mol Biochem Parasitol. 2022;248:111461. doi:10.1016/j.molbiopara.2022.111461
Ma W, Balta VA, Pan W, Rimer JD, Sullivan DJ, Vekilov PG. Nonclassical mechanisms to irreversibly suppress β-hematin crystal growth. Commun Biol. 2023;6(1):783. doi:10.1038/s42003-023-05046-z
Dondorp AM, Nosten F, Yi P, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361(5):455-467. doi:10.1056/NEJMoa0808859
Wellems TE, Sá JM, Su XZ, Connelly SV, Ellis AC. ‘Artemisinin resistance’: something new or old? something of a misnomer? Trends Parasitol. 2020;36(9):735-744. doi:10.1016/j.pt.2020.05.013
Auparakkitanon S, Wilairat P. Ring stage dormancy of Plasmodium falciparum tolerant to artemisinin and its analogues - a genetically regulated “Sleeping Beauty”. Int J Parasitol Drugs Drug Resist. 2023;21:61-64. doi:10.1016/j.ijpddr.2023.01.002
Looareesuwan S, Kyle DE, Viravan C, Vanijanonta S, Wilairatana P, Wernsdorfer WH. Clinical study of pyronaridine for the treatment of acute uncomplicated falciparum malaria in Thailand. Am J Trop Med Hyg. 1996;54(2):205-209. doi:10.4269/ajtmh.1996.54.205
Childs GE, Häusler B, Milhous W, et al. In vitro activity of pyronaridine against field isolates and reference clones of Plasmodium falciparum. Am J Trop Med Hyg. 1988;38(1):24-29. doi:10.4269/ajtmh.1988.38.24
Mahotorn K, Tan-Ariya P, Thita T, et al. In vitro sensitivity of pyronaridine in Thai isolates of Plasmodium falciparum. Am J Trop Med Hyg. 2018;98(1):51-56. doi:10.4269/ajtmh.17-0286
Madamet M, Briolant S, Amalvict R, et al. The Plasmodium falciparum chloroquine resistance transporter is associated with the ex vivo P. falciparum African parasite response to pyronaridine. Parasit Vectors. 2016;9:77. doi:10.1186/s13071-016-1358-z
Krogstad DJ, Gluzman IY, Kyle DE, et al. Efflux of chloroquine from Plasmodium falciparum: mechanism of chloroquine resistance. Science. 1987;238(4831):1283-1285. doi:10.1126/science.3317830
Wicht KJ, Mok S, Fidock DA. Molecular mechanisms of drug resistance in Plasmodium falciparum malaria. Annu Rev Microbiol. 2020;74:431-454. doi:10.1146/annurev-micro-020518-115546
Maughan SC, Pasternak M, Cairns N, et al. Plant homologs of the Plasmodium falciparum chloroquine-resistance transporter, PfCRT, are required for glutathione homeostasis and stress responses. Proc Natl Acad Sci USA. 2010;107(5):2331-2336. doi:10.1073/pnas.0913689107
Kimani SK, Shume JM. Antimalarial pyronaridine resistance may be associated with elevated MDR-1 gene expression profiles but not point mutation in Plasmodium berghei ANKA isolates. African J Biochem Res. 2020;14(4):102-111. doi:10.5897/AJBR2020.1097
van der Pluijm RW, Amaratunga C, Dhorda M, Dondorp AM. Triple artemisinin-based combination therapies for malaria - a new paradigm? Trends Parasitol. 2021;37(1):15-24. doi:10.1016/j.pt.2020.09.011
Ansbro MR, Itkin Z, Chen L, et al. Modulation of triple artemisinin-based combination therapy pharmacodynamics by Plasmodium falciparum genotype. ACS Pharmacol Transl Sci. 2020;3(6):1144-1157. doi:10.1021/acsptsci.0c00110
Pradines B, Tall A, Parzy D, et al. In-vitro activity of pyronaridine and amodiaquine against African isolates (Senegal) of Plasmodium falciparum in comparison with standard antimalarial agents. J Antimicrob Chemother. 1998;42(3):333-339. doi:10.1093/jac/42.3.333
Ringwald P, Eboumbou EC, Bickii J, Basco LK. In vitro activities of pyronaridine, alone and in combination with other antimalarial drugs, against Plasmodium falciparum. Antimicrob Agents Chemother. 1999;43(6):1525-1527. doi:10.1128/AAC.43.6.1525
Auparakkitanon S, Wilairat P. Antimalarial activity of concanamycin A alone and in combination with pyronaridine. Southeast Asian J Trop Med Public Health. 2006;37(4):619-621.
Xu C, Wong YK, Liao FL, Jiang T, Wang J, Tu Y. Is triple artemisinin-based combination therapy necessary for uncomplicated malaria? Lancet Infect Dis. 2022;22(5):585-586. doi:10.1016/S1473-3099(22)00208-0
Sereekhajornjaru N, Somboon C, Rattanajak R, Denny WA, Wilairat P, Auparakkitanon S. Comparison of hematin-targeting properties of pynacrine, an acridine analog of the benzonaphthyridine antimalarial pyronaridine. Acta Trop. 2014;140:181-183. doi:10.1016/j.actatropica.2014.09.002
Auparakkitanon S, Noonpakdee W, Ralph RK, Denny WA, Wilairat P. Antimalarial 9-anilinoacridine compounds directed at hematin. Antimicrob Agents Chemother. 2003;47(12):3708-3712. doi:10.1128/AAC.47.12.3708-3712.2003
Neumayr A, Kuenzli E. Quinacrine - The winding road from the most important antimalarial of its time to an indispensable antiparasitic (Orphan) drug of our days. Chimia (Aarau). 2023;77(9):574-576. doi:10.2533/chimia.2023.574
Fonte M, Tassi N, Gomes P, Teixeira C. Acridine-based antimalarials-from the very first synthetic antimalarial to recent developments. Molecules. 2021;26(3):600. doi:10.3390/molecules26030600
Scheindlin S. Something old... something blue. Mol Interv. 2008;8(6):268-273. doi:10.1124/mi.8.6.1
Lu G, Nagbanshi M, Goldau N, et al. Efficacy and safety of methylene blue in the treatment of malaria: a systematic review. BMC Med. 2018;16(1):59. doi:10.1186/s12916-018-1045-3
Ferreira FHDC, Pinto LR, Oliveira BA, Daniel LV, Navarro M, Delgado GYS. Analysis of the interaction of antimalarial agents with Plasmodium falciparum glutathione reductase through molecular mechanical calculations. J Mol Model. 2024;30(6):181. doi:10.1007/s00894-024-05968-3
Fabbri C, Ramos GQ, Baia-da-Silva DC, et al. The activity of methylene blue against asexual and sexual stages of Plasmodium vivax. Front Cell Infect Microbiol. 2023;13:1108366. doi:10.3389/fcimb.2023.1108366
Kumar A, Srivastava K, Kumar SR, Puri SK, Chauhan PM. Synthesis of new 4-aminoquinolines and quinoline-acridine hybrids as antimalarial agents. Bioorg Med Chem Lett. 2010;20(23):7059-7063. doi:10.1016/j.bmcl.2010.09.107
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 by the Authors. Licensee Ramathibodi Medical Journal.

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
















