A Comparative Study on Immunity Level and Adverse Effects in People who Received the AstraZeneca Vaccine or the Pfizer Vaccine Booster Dose via Intradermal versus Intramuscular Injection
Keywords:
Immunity Level, AstraZeneca Vaccine, Pfizer Vaccine, Intradermal, Intramuscular InjectionAbstract
This quasi-experimental research aimed to compare the levels of immunity and adverse effects in people receiving either the AstraZeneca or the Pfizer Covid-19 vaccine as a booster by intradermal (ID) or intramuscular (IM) injection. The sample was composed of 100 people, aged 18-60, who had previously received 2 consecutive doses of the Sinovac vaccine, then got boosted with a 3rd dose with either the AstraZeneca or the Pfizer vaccine boosters. Participants were divided into 4 groups of 25 people each. Outcomes were measured after 4 weeks. Data were analyzed using descriptive statistics, while differences between groups were compared with ANOVA and differences in pairs with Chef's statistical method.
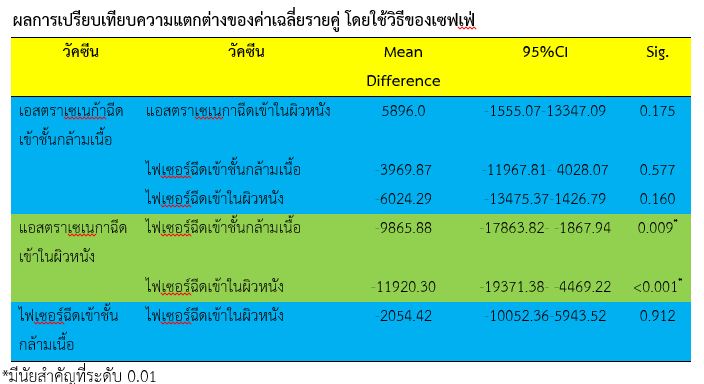
Results showed that, the highest mean level of immunity, after the 4th week of the experiment, was among the Pfizer ID (M= 20,916.16, S.D.= 12,503.87), the Pfizer IM (M= 18,861.74, S.D.= 10,866.45) and the AstraZeneca IM (M= 14,891.87, S.D.= 8,980.13), respectively. The lowest was the AstraZeneca ID (M= 8,995.86, S.D.= 7,827.69) while participants of the Pfizer IM and the Pfizer ID groups had significantly higher immunity than those in the AstraZeneca ID group. The most common level of side effects was measured among participants of the AstraZeneca IM group (76.67%), the Pfizer IM group (69.57%), and the AstraZeneca ID group (66.67%), respectively. The lowest level of side effects was observed among participants in the Pfizer ID group. There was a significant difference between the number of participants affected by side effects found in each vaccine type and injection technique.
In conclusion, after vaccination with the Pfizer IM and the Pfizer ID 3rd boosters, participants had a higher immunity level against COVID-19 than participants who had received AstraZeneca ID 3rd booster shot. Consequently, there must be ongoing research studies with repeated autoimmune examinations to assess which vaccines weaken the immune system faster.
References
Cao, Z. J., Wang, S. M., & Chen, Y. (2015). A randomized trial of multiple interventions for childhood obesity in China. American Journal of Preventive Medicine, 48(5), 552-560.
Cohen, J. (1988). Statistical power analysis for the behavioral sciences. Hillsdale, New Jersey: Lawrence Erlbaum Associates. Department of Medical Sciences. (2021). Immunity and safety testing of 3rd dose booster vaccine following 2 dose of sinovac in the general population. Retrieved March 2, 2022 from https://www.dmsc.moph.go.th/post-view/1305. (in Thai)
Faculty of Medicine Siriraj Hospital, Mahidol University. (2021). Siriraj releases research on COVID vaccine. Retrieved November 4, 2021 from https://www.si.mahidol.ac.th/th/ hotnewsdetail.asp?hn_id= 2691. (in Thai)
Jantarasuwan, S. & Buatoun, S. (2004). Research Methods in Social Science. Khon Kaen University. Khon Kaen. (in Thai)
Manmana, S., Iamsirithaworn, S., & Uttayamakul, S. (2020). Coronavirus-19 (COVID-19). Journal of Bamrasnaradura Infectious Diseases Institute, 14(2), 124-133.
Princ Hospital Suvarnabhumi. (2021). Injected into the skin. New hope. Spread the vaccine thoroughly. Stop the outbreak, Use less drugs, High immunity nearby, Injected into the muscle. Retrieved March 2, 2021 from https://www.princsuvarnabhumi.com/covid-dermal-injection. (in Thai)
Singhal, T. (2020). A review of coronavirus disease-2019. Indian J Pediatr, 87(4), 281–286. Today New. (2021). Results of safety studies and immune response from administer 3rd dose booster vaccination in persons who have received two doses of sinovac or ultraZeneca. Retrieved March 2, 2021 from https://workpointtoday.com/covid19-170/. (in Thai)
Wang, C., Horby, P. W., Hayden, F. G. & Gao, G. F. (2020). A novel coronavirus outbreak of global health concern. Lancet, 395, 470–473.
Worldmeters. (2021). World population. Retrieved January 14, 2021 from https://www.worldometers.info/th/. (in Thai)
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Boromarajonani College of Nursing, Nakhon Si Thammarat

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
บทความที่ได้รับการตีพิมพ์เป็นลิขสิทธิ์ของ วิทยาลัยพยาบาลบรมราชชนนี นครศรีธรรมราช
ข้อความที่ปรากฏในบทความแต่ละเรื่องในวารสารวิชาการเล่มนี้เป็นความคิดเห็นส่วนตัวของผู้เขียนแต่ละท่านไม่เกี่ยวข้องกับวิทยาลัยพยาบาลบรมราชชนนี นครศรีธรรมราช และบุคคลากรท่านอื่น ๆ ในวิทยาลัยฯ แต่อย่างใด ความรับผิดชอบองค์ประกอบทั้งหมดของบทความแต่ละเรื่องเป็นของผู้เขียนแต่ละท่าน หากมีความผิดพลาดใดๆ ผู้เขียนแต่ละท่านจะรับผิดชอบบทความของตนเองแต่ผู้เดียว




