Amisulpride: Medication for schizophrenia
Keywords:
amisulpride, antipsychotics, schizophreniaAbstract
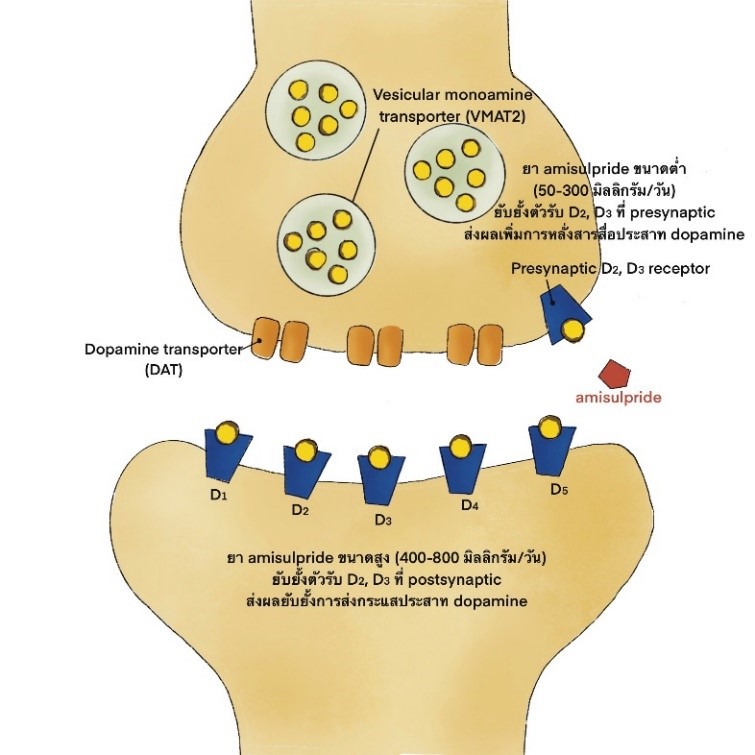
Amisulpride is a benzamide derivative which categorized as second generation antipsychotics that selectively blocks dopamine 2 and dopamine 3 receptors. The clinical efficacy of amisulpride to treat schizophrenia is superior to the first generation antipsychotics as well as non-inferior to the second generation antipsychotics. The recommended doses of amisulpride in schizophrenia patients with positive symptoms are higher than that of negative symptoms (400-800 and 50-300 mg/day, respectively). In terms of safety, the most common side effects of amisulpride are extrapyramidal symptoms and hyperprolactinemia, however the side effects are considered lower when compared to haloperidol and risperidone. Few metabolic side effect is found and the effects of adrenergic, cholinergic, and histamine blocking are still not reported. Moreover, the QT prolongation associated with cardiac sudden death from amisulpride overdose was reported. Therefore, the risk assessment should be performed prior to starting amisulpride, and closely monitoring after using medication.
References
Crismon ML, Argo TR, Buckley P. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, editors. Pharmacotherapy: a pathophysiological approach. 9th ed. New York: McGraw-Hill Education; 2014. p.1019-45.
Lacro JP, Farhadian S, Endow-Eyer RA. In: Alldredge BK, Corelli RL, Ernst ME, Guglielmo BJ, Jacobson PA, Kradjan WA, et al, editors. Koda Kimble and Young's applied therapeutics: the clinical use of drugs. 10th ed. Philadelphia: Lippincott Williams & Wilkins Kluwer business; 2014. p1921-48.
มาโนช หล่อตระกูล. บทที่ 11 โรคจิตเภทและโรคอื่น ๆ. ใน: มาโนช หล่อตระกูล, ปราโมทย์ สุคนิชย์, บรรณาธิการ. จิตเวชศาสตร์ รามาธิบดี. ฉบับพิมพ์ครั้งที่ 4. กรุงเทพฯ: ภาควิชาจิตเวชศาสตร์คณะแพทยศาสตร์โรงพยาบาลรามาธิบดี มหาวิทยาลัยมหิดล; 2558. หน้า 129-54.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed (DSM-5). Washington, DC: American Psychiatric Publishing; 2013. 87-123.
ถนอมพงษ์ เสถียรลัคนา. Pharmacotherapy of schizophrenia [อินเตอร์เน็ต]. ศูนย์การศึกษาต่อเนื่องทางเภสัชศาสตร์ (Center for Continuing Pharmaceutical Education); 2559 [สืบค้นเมื่อ 26 ตุลาคม 2564]. สืบค้นจาก: https://ccpe.pharmacycouncil.org/index.php?option=article_detail&subpage=article_detail&id=161
Ayano G. First generation antipsychotics: pharmacokinetics, pharmacodynamics, therapeutic effects and side effects: a review. RRJCHEM. 2016;5(3):53-63.
Divac N, Prostran M, Jakovcevski I, Cerovac N. Second-generation antipsychotics and extrapyramidal adverse effects. Biomed Res Int. 2014;2014:656370. doi:10.1155/2014/656370.
Mauri MC, Paletta S, Maffini M, Colasanti A, Dragogna F, Pace CD, et al. Clinical pharmacology of atypical antipsychotics: an update. EXCLI J. 2014;13:1163-91.
Amisulpride [Internet]. Drugs.com Content and Services Guide; 2021 [cited 2021 May 31]. Available from: https://www.drugs.com/international/amisulpride.html
Solian® ตรวจสอบการอนุญาต [อินเตอร์เน็ต]. สำนักงานคณะกรรมการอาหารและยา กระทรวงสาธารณสุข; [สืบค้นเมื่อ 26 ตุลาคม 2564]. สืบค้นจาก: https://porta.fda.moph.go.th/FDA_SEARCH_ALL/MAIN/SEARCH_CENTER_MAIN.aspx
Patteet L, Morrens M, Maudens KE, Niemegeers P, Sabbe B, Neels H. Therapeutic drug monitoring of common antipsychotics. Ther Drug Monit. 2012;34(6):629-51.
Solian® [package insert]. Thailand: Sanofi-Aventis (Thailand) Ltd; 2018.
Amisulpride - mechanism of action | Psychopharmacology | Clinical application [Internet]. Psychscenehub; 2020 [cited 2021 May 31]. Available from: https://psychscenehub.com/psychinsights/amisulpride-psychopharmacology/
Rang HP, Ritter JM, Flower RJ, Henderson G, editors. Rang & Dale's Pharmacology. 8th ed. China: Elsevier; 2016. P467-72.
Stahl SM. Drugs for psychosis and mood: unique actions at D3, D2, and D1 dopamine receptor subtypes. CNS Spectr. 2017;22(5):375-84.
Juruena MF, de Sena EP, de Oliveira IR. Specific mechanism of action of amisulpride in the treatment of schizophrenia and correlation with clinical response and tolerability. J Receptor Ligand Channel Res. 2011;4:49-55.
Basso AM, Gallagher KB, Bratcher NA, Brioni JD, Moreland RB, Hsieh GC, et al. Antidepressant-like effect of D(2/3) receptor-, but not D(4) receptor-activation in the rat forced swim test. Neuropsychopharmacology. 2005;30(7):1257–68.
Racagni G, Canonico PL, Ravizza L, Pani L, Amore M. Consensus on the use of substituted benzamides in psychiatric patients. Neuropsychobiology. 2004;50(2):134-43.
Abbas AI, Hedlund PB, Huang XP, Tran TB, Meltzer HY, Roth BL. Amisulpride is a potent 5-HT7 antagonist: relevance for antidepressant actions in vivo. Psychopharmacology (Berl). 2009;205(1):119- 28.
Biochemistry, Dopamine receptors [Internet]. StatPearls; 2020 [cited 2021 May 31]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538242/#_NBK538242_pubdet_
SmPC Solian 200mg Tablets [Internet]. EMC; 2021 [cited 2021 May 31]. Available from: https://www.medicines.org.uk/emc/product/4891/smpc#gref
Carrière P, Bonhomme D, Lempérière T. Amisulpride has a superior benefit/risk profile to haloperidol in schizophrenia: results of a multicentre, double-blind study (the Amisulpride Study Group). Eur Psychiatry. 2000;15(5):321-9.
Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373(9657):31-41.
Martin S, Lôo H, Peuskens J, Thirumalai S, Giudicelli A, Fleurot O, et al. A double-blind, randomised comparative trial of amisulpride versus olanzapine in the treatment of schizophrenia: short-term results at two months. Curr Med Res Opin. 2002;18(6):355-62.
Sechter D, Peuskens J, Fleurot O, Rein W, Lecrubier Y. Amisulpride vs. risperidone in chronic schizophrenia: results of a 6-month double-blind study. Neuropsychopharmacology. 2002;27(6):1071- 81.
Howard R, Cort E, Bradley R, Harper E, Kelly L, Bentham P, et al. Antipsychotic treatment of very late-onset schizophrenia-like psychosis (ATLAS): a randomised, controlled, double-blind trial. Lancet Psychiatry. 2018;5(7):553-63.
Australian Product Information – SOLIAN (amisulpride) tablets and solution [Internet]. Sanofi-Aventis Australia; 2020 [cited 2021 May 31]. Available from: http://www.guildlink.com.au/gc/ws/sw/pi.cfm?product=swpsolia11020
Odhejo YI, Jafri A, Mekala HM, Hassan M, Khan AM, Dar SK, et al. Safety and efficacy of antipsychotics in pregnancy and lactation. J Alcohol Drug Depend. 2017;5(3):1-7.
Are atypical antipsychotics safe during breast feeding? [Internet]. UK medicines information pharmacists; 2013 [cited 2021 May 31]. Available from: https://www.southstaffordshirejointformulary.nhs.uk/docs/apg/Central-Nervous-
System/Are%20atypical%20antipsychotics%20safe%20during%20breast%20feeding.pdf
Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-62.
Liang Y, Cao C, Zhu C, Wang C, Zane C, Dong F, et al. The effectiveness and safety of amisulpride in Chinese patients with schizophrenia: an 8-week, prospective, open-label, multicenter, single arm study. Asia Pac Psychiatry. 2016;8(3)241-4.
Bergemann N, Abu-Tair F, Kress KR, Parzer P, Kopitz J. Increase in plasma concentration of amisulpride after addition of concomitant lithium. J Clin Psychopharmacol. 2007;27(5):546–9.
Sparshatt A, Taylor D, Patel MX, Kapur S. Amisulpride - dose, plasma concentration, occupancy and response: implications for therapeutic drug monitoring: therapeutic drug monitoring of amisulpride. Acta Psychiatr Scand. 2009;120(6):416–28.
Practice guideline for the treatment of patients with schizophrenia. 3rd ed. [Internet]. American Psychiatric Association; 2021 [cited 2021 May 28]. Available from: https://psychiatryonline.org/doi/book/10.1176/appi.books.9780890424841
Barnes TRE, Drake R, Paton C, Cooper SJ, Deakin B, Ferrier IN, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharm. 2020;34(1):3–78.
Stahl SM. Stahl’s Essential Psychopharmacology. 6th ed. United Kingdom: Clays,St Ives plc; 2017. p17- 24.
Downloads
Published
How to Cite
Issue
Section
License
ข้อความภายในบทความที่ตีพิมพ์ในวารสารเภสัชกรรมโรงพยาบาลทั้งหมด รวมถึงรูปภาพประกอบ ตาราง เป็นลิขสิทธิ์ของสมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย) การนำเนื้อหา ข้อความหรือข้อคิดเห็น รูปภาพ ตาราง ของบทความไปจัดพิมพ์เผยแพร่ในรูปแบบต่าง ๆ เพื่อใช้ประโยชน์ในเชิงพาณิชย์ ต้องได้รับอนุญาตจากกองบรรณาธิการวารสาร (สมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย)) อย่างเป็นลายลักษณ์อักษร
สมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย) อนุญาตให้สามารถนำไฟล์บทความไปใช้ประโยชน์และเผยแพร่ต่อได้ โดยอยู่ภายใต้เงื่อนไขสัญญาอนุญาตครีเอทีฟคอมมอน (Creative Commons License: CC) โดย ต้องแสดงที่มาจากวารสาร – ไม่ใช้เพื่อการค้า – ห้ามแก้ไขดัดแปลง, Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0)
ข้อความที่ปรากฏในบทความในวารสารเป็นความคิดเห็นส่วนตัวของผู้เขียนแต่ละท่านไม่เกี่ยวข้องกับสมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย) และบุคลากรในสมาคมฯ แต่อย่างใด ความรับผิดชอบองค์ประกอบทั้งหมดของบทความแต่ละเรื่องเป็นของผู้เขียนแต่ละท่าน หากมีความผิดพลาดใด ๆ ผู้เขียนแต่ละท่านจะรับผิดชอบบทความของตนเอง ตลอดจนความรับผิดชอบด้านเนื้อหาและการตรวจร่างบทความเป็นของผู้เขียน ไม่เกี่ยวข้องกับกองบรรณาธิการ


.png)

