Voriconazole-induced CNS Toxicity: A Case Study
Keywords:
voriconazole, CNS toxicity, central nervous system toxicity, adverse drug reactionAbstract
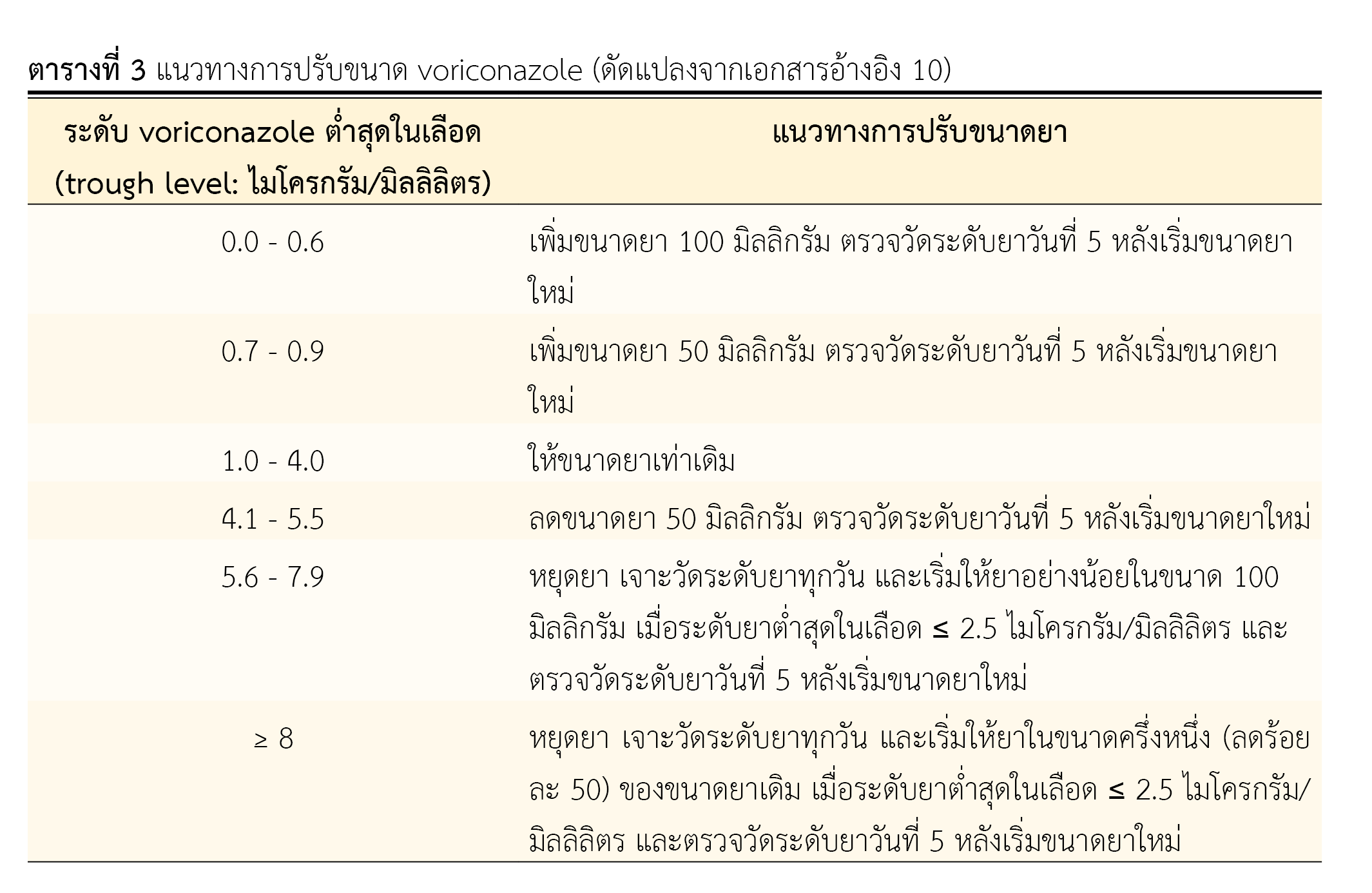
Voriconazole-induced central nervous system (CNS) toxicity is a common adverse drug reaction (ADR). It is associated with voriconazole plasma level higher than normal trough concentrations and other factors decreasing elimination of voriconazole. This CNS toxicity can be prevented by prescribing the recommended dose of voriconazole, on weight-based dosing. monitoring liver function test, and monitoring therapeutic drug level of voriconazole. Reduction or discontinuation of voriconazole could alleviate this ADR. In addition, the co-precipitating factor of voriconazole-induced CNS toxicity should be resolved. This is a case study of a 40-years old woman with B-cell acute lymphoblastic leukemia (B-ALL) developing confusion, disorganized speech symptoms, and hallucinations after three days of oral administration of voriconazole at 200 mg every 12 hours (4.7 mg/kg) for treatment of invasive pulmonary aspergillosis. Voriconazole-induced CNS toxicity was diagnosed and pharmacist was consulted. On reviewing the physical examination, the laboratory tests, and the medication history, there were evidences of a little bit higher blood level of voriconazole than recommended level and abnormal liver function test. These led to high blood level of voriconazole and induced CNS toxicity.
This report indicates the importance of preventing voriconazole-induced CNS toxicity. Pharmacist can play role in this prevention by screening high-risk patients, reviewing administered dose, and co-administered drugs, identifying drug interactions, consulting to monitor liver function test and therapeutic drug level, assessing for causes and co-precipitating factors leading to voriconazole-induced CNS toxicity.
References
Patterson TF, Thompson GR, Denning DW, Fishman JA, Hadley S, Herbrecht R, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;63:20-3.
Vfend™ [package insert]. Pfizer (Thailand) limited; 2016.
Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24:e1-e38. doi: 10.1016/j.cmi.2018.01.002. PMID: 29544767.
Bayhan GI, Garipardic M, Karaman K, Akbayram S. Voriconazole-associated visual disturbances and hallucinations. Cutan Ocul Toxicol. 2015;35(1):80-2.
Demir SÖ, Atici S, Akkoç G, Yakut N, EkizoLlu NB, Eralp EE, et al. Neurologic adverse events associated with voriconazole therapy: report of two pediatric cases. Case Rep Infect Dis. 2016;2016:3989070. doi: 10.1155/2016/3989070. PMID: 27313918.
Levine MT, Chandrasekar PH. Adverse effects of voriconazole: over a decade of use. Clin transplant. 2016;30:1377-86.
Jansen JW, Sen SK, Moenster RP. Elevated voriconazole level associated with hallucinations and suicidal ideation: a case report. Open Forum Infect Dis. 2017;4(1):ofw215. doi: 10.1093/ofid/ofw215. PMID: 28480228.
Zonios DI, Banacloche JG, Childs R, Bennett JE. Hallucinations during voriconazole therapy. Clin infect dis. 2008;47(1):e7– e10.
Imhof A, Schaer DJ, Schneemann M, Laffer R, Schanz U. Neurological adverse events to voriconazole: evidence for therapeutic drug monitoring [Abstract]. Int J Infect Dis. 2006;10(s1):S62.
Perreault S, McManus D, Anderson A, Lin T, Ruggero M, Topal JE. Evaluating a voriconazole dose modification guideline to optimize dosing in patients with hematologic malignancies. J Oncol Pharm Practice. 2019;25(6):1305–11.
Moriyama B, Obeng AO, Barbarino J, Penzak SR, Henning S, Scot SA. Clinical pharmacogenetics implementation consortium (CPIC) guidelines for CYP2C19 and voriconazole therapy. Clin Pharmacol Ther. 2017;102(1);45-51.
อาภรณี ไชยาคำ, เด่นพงศ์ พัฒนเศรษฐานนท์, ศิริลักษณ์ ใจซื่อ, บรรณาธิการ. การประยุกต์เภสัชจลนศาสตร์ทางคลินิก. พิมพ์ครั้งที่ 1. ขอนแก่น: โรงพิมพ์คลังนานาวิทยา; 2556.
Lin XB, Huang F, Tong L, Xia YZ, Wu JJ, Li J, et al. Pharmacokinetics of intravenous voriconazole in patients with liver dysfunction: A prospective study in the intensive care unit. Int J Infect Dis. 2020;93:345–52.
Downloads
Published
How to Cite
Issue
Section
License
ข้อความภายในบทความที่ตีพิมพ์ในวารสารเภสัชกรรมโรงพยาบาลทั้งหมด รวมถึงรูปภาพประกอบ ตาราง เป็นลิขสิทธิ์ของสมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย) การนำเนื้อหา ข้อความหรือข้อคิดเห็น รูปภาพ ตาราง ของบทความไปจัดพิมพ์เผยแพร่ในรูปแบบต่าง ๆ เพื่อใช้ประโยชน์ในเชิงพาณิชย์ ต้องได้รับอนุญาตจากกองบรรณาธิการวารสาร (สมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย)) อย่างเป็นลายลักษณ์อักษร
สมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย) อนุญาตให้สามารถนำไฟล์บทความไปใช้ประโยชน์และเผยแพร่ต่อได้ โดยอยู่ภายใต้เงื่อนไขสัญญาอนุญาตครีเอทีฟคอมมอน (Creative Commons License: CC) โดย ต้องแสดงที่มาจากวารสาร – ไม่ใช้เพื่อการค้า – ห้ามแก้ไขดัดแปลง, Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0)
ข้อความที่ปรากฏในบทความในวารสารเป็นความคิดเห็นส่วนตัวของผู้เขียนแต่ละท่านไม่เกี่ยวข้องกับสมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย) และบุคลากรในสมาคมฯ แต่อย่างใด ความรับผิดชอบองค์ประกอบทั้งหมดของบทความแต่ละเรื่องเป็นของผู้เขียนแต่ละท่าน หากมีความผิดพลาดใด ๆ ผู้เขียนแต่ละท่านจะรับผิดชอบบทความของตนเอง ตลอดจนความรับผิดชอบด้านเนื้อหาและการตรวจร่างบทความเป็นของผู้เขียน ไม่เกี่ยวข้องกับกองบรรณาธิการ


.png)

