กลุ่มโรควงจรยูเรียบกพร่อง
คำสำคัญ:
กลุ่มโรควงจรยูเรียบกพร่อง, ภาวะแอมโมเนียในเลือดสูง, sodium benzoate, arginineบทคัดย่อ
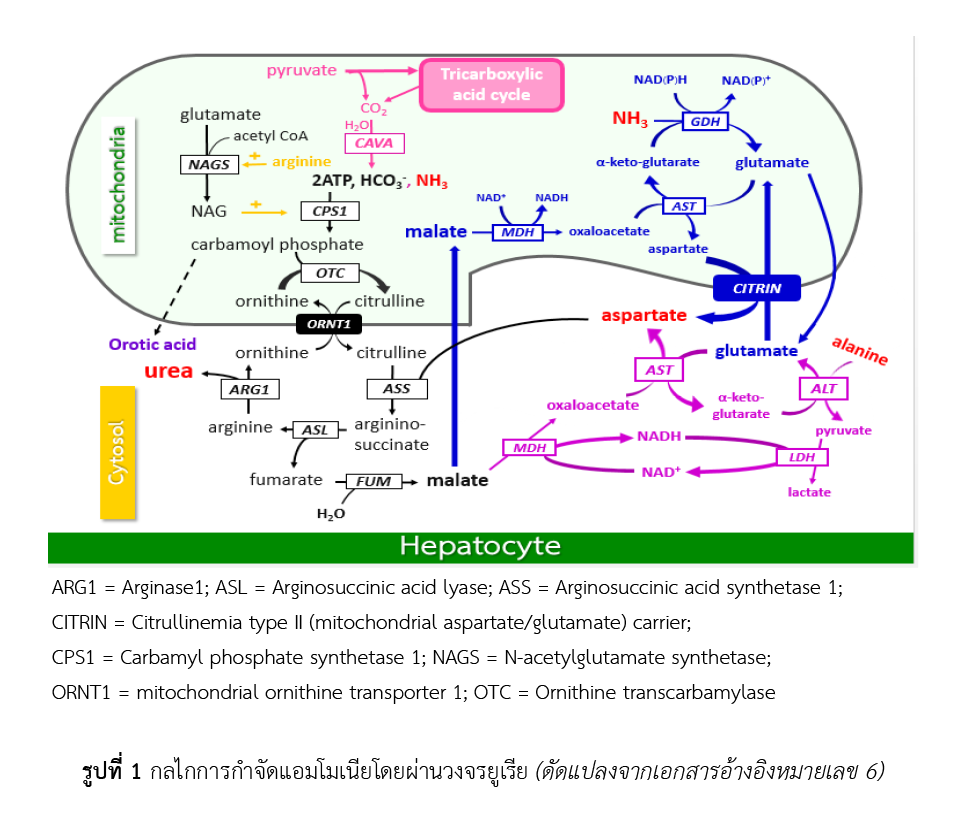
กลุ่มโรควงจรยูเรียบกพร่อง คือ กลุ่มโรคพันธุกรรมเมแทบอลิกที่มีอุบัติการณ์โดยประมาณที่ 1 : 35,000 ของประชากรทารกแรกเกิดในสหรัฐอเมริกา ผู้ป่วยกลุ่มโรควงจรยูเรียบกพร่องในทารกแรกเกิดร้อยละ 50 พบว่ามีภาวะแอมโมเนียในเลือดสูง และมีอัตราการเสียชีวิตสูงถึงร้อยละ 25 - 50 ตามมาด้วยอาการทางระบบประสาทอย่างรุนแรงในผู้รอดชีวิตหากไม่ได้รับการรักษาอย่างทันท่วงที กลุ่มโรควงจรยูเรียบกพร่องเกิดจากความผิดปกติของเอนไซม์ 1 ใน 6 ชนิด หรือโปรตีนขนส่ง 2 ตัว ที่เกี่ยวข้องในวงจรยูเรีย ทำให้ร่างกายไม่สามารถเปลี่ยนแอมโมเนียให้อยู่ในรูปของยูเรียเพื่อขับออกผ่านไตได้ตามปกติ เกิดความเป็นพิษจากภาวะแอมโมเนียในเลือดสูงต่อระบบต่าง ๆ ภายในร่างกาย ในการรักษากลุ่มโรควงจรยูเรียบกพร่อง ควรจำกัดการรับประทานโปรตีนที่จะสลายไปเป็นแอมโมเนีย และเลือกรับประทานเฉพาะโปรตีนคุณภาพสูงร่วมกับการรับประทานยาซึ่งแบ่งเป็น 2 กลุ่ม ตามกลไกการออกฤทธิ์ กลุ่มแรกคือกลุ่มยาที่เป็น nitrogen scavengers ได้แก่ sodium benzoate, sodium phenylbutyrate, sodium phenylacetate และ glycerol phenylbutyrate กลุ่มที่สองคือ กลุ่มยาที่เป็น deficient metabolites ได้แก่ L-arginine, L-citrulline, และ carbamylglutamate โดยมีวัตถุประสงค์เพื่อควบคุมระบบเมแทบอลิซึมที่จะส่งผลต่อการเจริญเติบโตหรือพัฒนาการของร่างกายให้เป็นปกติ และเพื่อป้องกันโรคแทรกซ้อนที่อาจเกิดขึ้นในอนาคต
เอกสารอ้างอิง
พรสวรรค์ วสันต์. โรคพันธุกรรมเมแทบอลิก (Inherited metabolic disorders). ใน: กิตติ อังศุสิงห์, นวลอนงค์ วิศิษฎสุนทร, อัจฉรา สัมบุณณานนท์, วาณี วิสุทธิ์เสรีวงศ์, กฤตย์วิกรม ดรุงค์พิศิษฐ์กลุ, บรรณาธิการ. กุมารเวชปฏิบัติทันยุค. กรุงเทพมหานคร: ชวนพิมพ์; 2542. หน้า 233-49.
Scaglia F, Brunetti-Pierri N, Kleppe S, Marini J, Carter S, Garlick P, et al. Clinical consequences of urea cycle enzyme deficiencies and potential links to arginine and nitric oxide metabolism. J Nutr. 2004;134(10):2775S-82S.
Mew NA, Simpson KL, Gropman AL, Lanpher BC, Chapman KA, Summar ML. Urea cycle disorders overview. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle: University of Washington; 2003 [cited 2021 Jun 27]. 1-21. Available from https://www.ncbi.nlm.nih.gov/books/NBK1217/pdf/Bookshelf _NBK1217.pdf
Redant S, Empain A, Mugisha A, Kamgang P, Attou R, Honoré P, et al. Management of late onset urea cycle disorders - a remaining challenge for the intensivist? Ann Intensive Care. 2021;11(1):1-10.
พิสิฏฐ์ ประพันธ์วัฒนะ. บทที่ 12 ความผิดปกติแต่กำเนิดของกระบวนการเมแทบอลิซึม. ใน: เมแทบอลิซึมและโภชนาการ. [อินเทอร์เน็ต]. กรุงเทพมหานคร: ภาควิชาชีวเคมี คณะแพทยศาสตร์ จุฬาลงกรณ์มหาวิทยาลัย; 2555 [สืบค้น 3 เมษายน 2564]. เข้าถึงได้จาก: http://biochem.md.chula.ac.th/Data/PDF%20files/IEM2555.pdf.
Häberle J, Burlina A, Chakrapani A, Dixon M, Karall D, Lindner M, et al. Suggested guidelines for the diagnosis and management of urea cycle disorders: First revision. J Inherit Metab Dis. 2019;42(6):1192-230.
Stone WL, Basit H, Jaishankar G. Urea cycle disorders [Internet]. Bethesda, MD: National Center for Biotechnology Information; 2021 [cited 2021 Jun 27]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482363
Daniel LI, Angulo-Cruz I, Sánchez-Abdon L, Patricio-Martínez A. Disturbance of the glutamate-glutamine cycle, secondary to hepatic damage, compromises memory function. Front Neurosci. 2021;15:1-16.
Lerma EV. Blood Urea Nitrogen (BUN) [Internet]. New York: Medscape; 2019 [cited 2021 Jun 27]. Available from: https://emedicine.medscape.com/article/2073979-overview
Barmore W, Azad F, Stone WL. Physiology, urea cycle [Internet]. Treasure Island: StatPearls; 2021. [cited 2021 Jun 27]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513323
Batshaw M, Monahan P. Treatment of urea cycle disorders. Enzyme. 1987;38(1-4):242-50.
Maines E, Urru S, Burri E, Piccoli G, Pedrolli A, Pasqualini A, et al. Formulation and clinical evaluation of sodium benzoate oral solution for the treatment of urea cycle disorders in pediatric patients. AAPS PharmSciTech. 2020;21(100):1-8.
Balado A, Fernández A, Barrientos I, Montero J, Rey I, Ferreiro A, et al. 3PC-005 Stability study of a 10% sodium benzoate oral solution. Section 3: Production and compounding. Eur J Hosp Pharm. 2019;26(Suppl 1):A39.
Hussein Q. Paediatric formulations of L-arginine for the use in urea cycle disorders [Internet]. Düsseldorf: Heinrich Heine University; 2009 [cited 2021 Jun 27]. Available from: https://docserv.uni-duesseldorf.de/servlets/DerivateServlet/Derivate-13784/Promotion,%20Qaed%20Abdul%20Hussein.pdf
The United States Pharmacopeial Convention. General Chapters <795> Pharmaceutical Compounding – Nonsterile Preparations [Internet]. Maryland: The United States Pharmacopeial Convention; updated 2020 May 1 [cited 2021 Jul 13]. Available from: https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/revisions/gc-795-rb-notice-20200424.pdf
Bawaskar HS, Bawaskar PH, Bawaskar PH. Chinese restaurant syndrome. Indian J Crit Care Med. 2017;21(1):49–50.
Häberle J, Boddaert N, Burlina A, Chakrapani A, Dixon M, Huemer M, et al. Suggested guidelines for the diagnosis and management of urea cycle disorders. Orphanet J Rare Dis. 2012;7(1):32.
The United States Pharmacopeial Convention. General Chapters <795> Pharmaceutical Compounding - Nonsterile Preparations [Internet]. Maryland: The United States Pharmacopeial Convention; updated 2019 Dec 1 [cited 2021 Jul 13]. Available from: https://www.uspnf.com/sites/default /files/usp_pdf/EN/USPNF/revisions/gc-795-postponement-rb-notice-20191122.pdf
ดาวน์โหลด
เผยแพร่แล้ว
รูปแบบการอ้างอิง
ฉบับ
ประเภทบทความ
สัญญาอนุญาต
ลิขสิทธิ์ (c) 2022 สมาคมเภสัชกรรมโรงพยาบาล(ประเทศไทย)

อนุญาตภายใต้เงื่อนไข Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ข้อความภายในบทความที่ตีพิมพ์ในวารสารเภสัชกรรมโรงพยาบาลทั้งหมด รวมถึงรูปภาพประกอบ ตาราง เป็นลิขสิทธิ์ของสมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย) การนำเนื้อหา ข้อความหรือข้อคิดเห็น รูปภาพ ตาราง ของบทความไปจัดพิมพ์เผยแพร่ในรูปแบบต่าง ๆ เพื่อใช้ประโยชน์ในเชิงพาณิชย์ ต้องได้รับอนุญาตจากกองบรรณาธิการวารสาร (สมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย)) อย่างเป็นลายลักษณ์อักษร
สมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย) อนุญาตให้สามารถนำไฟล์บทความไปใช้ประโยชน์และเผยแพร่ต่อได้ โดยอยู่ภายใต้เงื่อนไขสัญญาอนุญาตครีเอทีฟคอมมอน (Creative Commons License: CC) โดย ต้องแสดงที่มาจากวารสาร – ไม่ใช้เพื่อการค้า – ห้ามแก้ไขดัดแปลง, Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0)
ข้อความที่ปรากฏในบทความในวารสารเป็นความคิดเห็นส่วนตัวของผู้เขียนแต่ละท่านไม่เกี่ยวข้องกับสมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย) และบุคลากรในสมาคมฯ แต่อย่างใด ความรับผิดชอบองค์ประกอบทั้งหมดของบทความแต่ละเรื่องเป็นของผู้เขียนแต่ละท่าน หากมีความผิดพลาดใด ๆ ผู้เขียนแต่ละท่านจะรับผิดชอบบทความของตนเอง ตลอดจนความรับผิดชอบด้านเนื้อหาและการตรวจร่างบทความเป็นของผู้เขียน ไม่เกี่ยวข้องกับกองบรรณาธิการ


.png)

