L-Carnitine Supplementation in Pediatric Patients with End-Stage Renal Disease on Peritoneal Dialysis
Keywords:
L-carnitine, carnitine deficiency, peritoneal dialysis, pediatric patients, end-stage renal diseaseAbstract
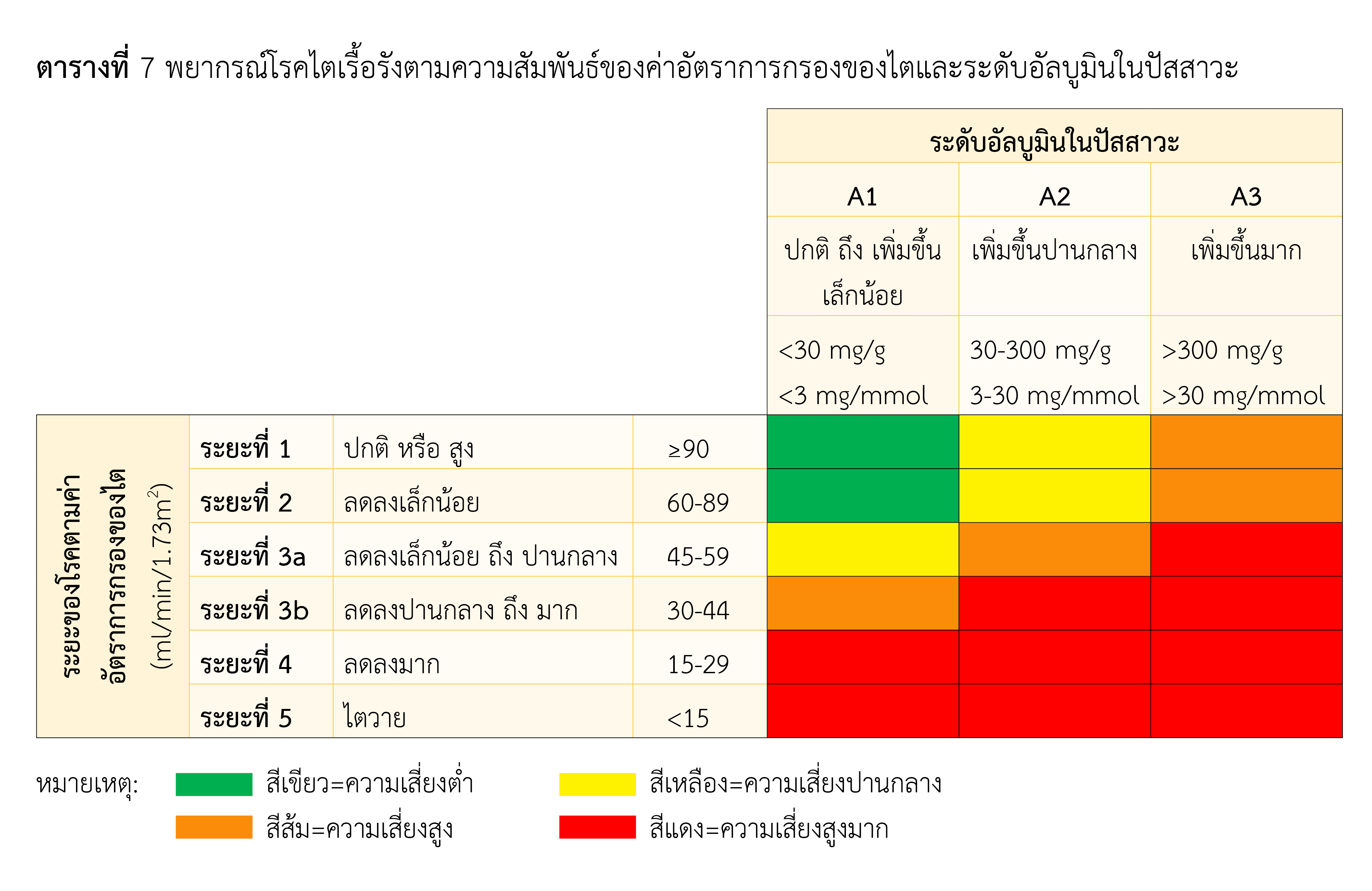
Kidney disease is a serious health problem, and number of patients is gradually climbing. Cause of renal dysfunction in children varies with age. In young children, it is mostly caused by structural or genetic abnormalities. However, Infections and other illnesses are the most common causes in older children. As a consequence, function of kidney is declined and has an impact on waste disposal, water and mineral balance in the body. Carnitine level is also affected and may result in carnitine deficiency with disorder of the body. This deficiency in patients with chronic kidney disease are found and known to be caused by inadequate consumption of carnitine-containing food, reduced capacity of kidney to synthesis carnitine, loss of free carnitine during hemodialysis or peritoneal dialysis, and loss of acyl carnitine by renal excretion. L-carnitine is supplemented as it is an active form but at present, no recommendation for initial dose of L-carnitine in pediatric patients with kidney disease who are on peritoneal dialysis. However, the dose of L-carnitine for those who are on hemodialysis is 10–20 mg/kg after hemodialysis and L-carnitine is administered by injection. In Thailand, L-carnitine is available as oral dosage form only, and its commercial product is food supplement. Nowadays, study on efficacy of L-carnitine in end-stage renal disease is limited, and further study is needed.
References
Gulewitsch W, Krimberg R. Zur Kenntnis der Extraktivstoffe der Muskeln. II. Mitteilung. Über das Carnitin. 1905;45(3-4):326-30.
Bremer J. Carnitine--metabolism and functions. Physiol Rev. 1983;63(4):1420-80.
Reuter SE, Evans AM. Carnitine and acylcarnitines. Clin Pharmacokine. 2012;51(9):553-72.
National Center for Biotechnology Information. PubChem Compound Summary for CID 288, Carnitine [Internet]. National Library of Medicine (US); 2021 [cited 2021 August 2]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Carnitine.
Brass EP. Pharmacokinetic considerations for the therapeutic use of carnitine in hemodialysis patients. Clin Ther. 1995;17(2):176-85; discussion 5.
Rebouche CJ, Chenard CA. Metabolic fate of dietary carnitine in human adults: identification and quantification of urinary and fecal metabolites. J Nutr. 1991;121(4):539-46.
Rebouche CJ. Carnitine function and requirements during the life cycle. FASEB J. 1992;6(15):3379-86.
Strijbis K, Vaz FM, Distel B. Enzymology of the carnitine biosynthesis pathway. IUBMB Life. 2010;62(5):357-62.
Muthuramu IS, Neha & Amin, Md Ruhul & Geest, Bart. Role of lipids and lipoproteins in myocardial biology and in the development of heart failure. Clin Lipidol. 2015;10:329-42.
Houten SM, Wanders RJA. A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J Inherit Metab Dis. 2010;33(5):469-77.
คณะอนุกรรมการลงทะเบียนการบำบัดทดแทนไต (TRT). ข้อมูลการบำบัดทดแทนไตในประเทศไทย พ.ศ. 2563: สมาคมโรคไตแห่งประเทศไทย; 2563.
Ingsathit A, Thakkinstian A, Chaiprasert A, Sangthawan P, Gojaseni P, Kiattisunthorn K, et al. Prevalence and risk factors of chronic kidney disease in the Thai adult population: Thai SEEK study. Nephrol Dial Transplant. 2009;25(5):1567-75.
Becherucci F, Roperto RM, Materassi M, Romagnani P. Chronic kidney disease in children. Clin Kidney J. 2016;9(4):583-91.
Kidney Disease: Improving Global Outcomes (KDIGO) Chapter 1: definition and classification of CKD. Kidney Int Suppl (2011). 2013;3(1):19–62.
คณะกรรมการผู้เชี่ยวชาญอนุสาขาวิชาโรคไตเด็ก และชมรมโรคไตเด็กแห่งประเทศไทย. แนวทางเวชปฏิบัติโรคไตเรื้อรังในทารกแรกเกิดถึงเด็กอายุ 18 ปี [อินเตอร์เน็ต]. 2562 [ เข้าถึงเมื่อ 2 ส.ค. 2564]. เข้าถึงได้จาก: http://www.thaipediatrics.org/Media/media-20190614093015.pdf
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-12.
สมาคมโรคไตแห่งประเทศไทย. สูตรคำนวณการวัดระดับครีเอตินินในเลือดให้ได้มาตรฐานเพื่อประเมินการทำงานของไตด้วยค่า estimated glomerular filtration rate (eGFR) [อินเตอร์เน็ต]. สมาคมโรคไตแห่งประเทศไทย; 2016 [เข้าถึงเมื่อ 2 ส.ค. 2564]. เข้าถึงได้จาก: http://doh.hpc.go.th/data/HL/eGFR.pdf
พงศธร คชเสนี. การบำบัดทดแทนไตในปัจจุบัน [อินเตอร์เน็ต]. 2020 [เข้าถึงเมื่อ 8 ส.ค. 2564]. เข้าถึงได้จาก: https://www.nephrothai.org/wp-content/uploads/2020/08/การบำบัดทดแทนไตในปัจจุบัน.pdf
ศูนย์รับบริจาคอวัยวะสภากาชาดไทย. รายงานประจำปี 2563 [อินเตอร์เน็ต]. 2563 [เข้าถึงเมื่อ 2 ส.ค. 2564]. เข้าถึงได้จาก: https://www.organdonate.in.th/assets/files/odc2563.pdf.
Shimizu S, Takashima H, Tei R, Furukawa T, Okamura M, Kitai M, et al. Prevalence of carnitine deficiency and decreased carnitine levels in patients on peritoneal dialysis. Nutrients. 2019;11(11):2645.
Bonomini M, Di Liberato L, Zammit V, Arduini A. Current opinion on usage of L-carnitine in end-stage renal disease patients on peritoneal dialysis. Molecules. 2019;24(19):3449.
Naseri M, Mottaghi Moghadam Shahri H, Horri M, Esmaeeli M, Ghaneh Sherbaf F, Jahanshahi S, et al. Absolute and relative carnitine deficiency in patients on hemodialysis and peritoneal dialysis. Iran J Kidney Dis. 2016;10(1):36-43.
Burwinkel B, Kreuder J, Schweitzer S, Vorgerd M, Gempel K, Gerbitz KD, et al. Carnitine transporter OCTN2 mutations in systemic primary carnitine deficiency: a novel Arg169Gln mutation and a recurrent Arg282ter mutation associated with an unconventional splicing abnormality. Biochem Biophys Res Commun. 1999;261(2):484-7.
Erguven M, Yilmaz O, Koc S, Caki S, Ayhan Y, Donmez M, et al. A case of early diagnosed carnitine deficiency presenting with respiratory symptoms. Ann Nutr Metab. 2007;51(4):331-4.
Stanley CA. Carnitine deficiency disorders in children. Ann N Y Acad Sci. 2004;1033:42-51.
Winter SC. Treatment of carnitine deficiency. J Inherit Metab Dis. 2003;26(2-3):171-80.
Khositseth A, Jirasakpisarn S, Pakakasama S, Choubtuym L, Wattanasirichaigoon D. Carnitine levels and cardiac functions in children with solid malignancies receiving doxorubicin therapy. Indian J Med Paediatr Oncol. 2011;32(1):38-42.
Kidney Disease Outcomes Quality Initiative (KDOQI). KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 update. Executive summary. Am J Kidney Dis. 2009;53(3 Suppl 2):S11-104.
Morgans HA, Chadha V, Warady BA. The role of carnitine in maintenance dialysis therapy. Pediatr Nephrol. 2021;36(8):2545-51.
Bieber LL. Carnitine. Annu Rev Biochem. 1988;57:261-83.
Kletzmayr J, Mayer G, Legenstein E, Heinz-Peer G, Leitha T, Hörl WH, et al. Anemia and carnitine supplementation in hemodialyzed patients. Kidney Int Suppl. 1999;69:S93-106.
Matsumura M, Hatakeyama S, Koni I, Mabuchi H, Muramoto H. Correlation between serum carnitine levels and erythrocyte osmotic fragility in hemodialysis patients. Nephron. 1996;72(4):574-8.
Wang Z-Y, Liu Y-Y, Liu G-H, Lu H-B, Mao C-Y. L-Carnitine and heart disease. Life Sciences. 2018;194:88-97.
Schreiber B. Levocarnitine and dialysis: a review. Nutr Clin Pract. 2005;20(2):218-43.
Longo N, Frigeni M, Pasquali M. Carnitine transport and fatty acid oxidation. Biochim Biophys Acta. 2016;1863(10):2422-35.
Eknoyan G, Latos DL, Lindberg J. Practice recommendations for the use of L-carnitine in dialysis-related carnitine disorder National Kidney Foundation Carnitine Consensus Conference. Am J Kidney Dis. 2003;41(4):868-76.
Sobel S. Approval of efficacy supplement for use in end stage renal disease [Internet]. Carnitor (levocarnitine injection); 1999 [cited 2021 Aug 27]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/99/20182S6_carnitor%20injection_medr.pdf
Sanchez-Niño MD, Ortiz A. Differential effects of oral and intravenous L-carnitine on serum lipids: is the microbiota the answer? Clin Kidney J. 2014;7(5):437-41.
Fukami K, Yamagishi S, Sakai K, Nasu M, Okuda S. Effects of switching from oral administration to intravenous injection of L-carnitine on lipid metabolism in hemodialysis patients. Clin Kidney J. 2014;7(5):470-4.
Evans A. Dialysis-related carnitine disorder and levocarnitine pharmacology. Am J Kidney Dis. 2003;41:S13-26.
Leadiant Biosciences. Carnitor (levocarnitine) injection [Internet]. Leadiant Biosciences; 2018 [cited 2021 Aug 28]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/020182s015lbl.pdf
Lilien MR, Duran M, Quak JM, Frankhuisen JJ, Schröder CH. Oral L-carnitine does not decrease erythropoietin requirement in pediatric dialysis. Pediatr Nephrol. 2000;15(1-2):17-20.
Verrina E, Caruso U, Calevo MG, Emma F, Sorino P, De Palo T, et al. Effect of carnitine supplementation on lipid profile and anemia in children on chronic dialysis. Pediatr Nephrol. 2007;22(5):727-33.
Koşan C, Sever L, Arisoy N, Calişkan S, Kasapçopur O. Carnitine supplementation improves apolipoprotein B levels in pediatric peritoneal dialysis patients. Pediatr Nephrol. 2003;18(11):1184-8.
Warady BA, Borum P, Stall C, Millspaugh J, Taggart E, Lum G. Carnitine status of pediatric patients on continuous ambulatory peritoneal dialysis. Am J Nephrol. 1990;10(2):109-14.
Wolters Kluwer. Carnitine supplements (Levocarnitine): Pediatric drug information. UpToDate [Internet]. 2022 [cited 2021 Aug 28]. Available from: https://www.uptodate.com/contents/carnitine-supplements-levocarnitine-pediatric-drug-information?search=levocarnitine&source=panel_search_result&selectedTitle=2~36&usage_type=panel&kp_tab=drug_pediatric&display_rank=1
Secretary-General of Food and Drug Administration. Requirement for use of amino acids as active ingredients in food supplements [Internet]. Thai Food and Drug Administration; 2006 [cited 2021 Aug 28]. Available from: http://food.fda.moph.go.th/law/data/announ_fda/English/20%20Use%20of%20amino%20acids%20(with%20annex).pdf
Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol. 2006;16(6):710-5.
Malaguarnera M. Carnitine derivatives: clinical usefulness. Curr Opin Gastroenterol. 2012;28(2):166-76. doi: 10.1097/MOG.0b013e3283505a3b.
United States Food and Drug Administration. Carnitor (levocarnitine) Tablets (330 mg), Oral Solution (1g per 10 mL multidose), Sugar-Free Oral Solution (1 g per 10 mL multidose) [Internet]. 2018 [cited 2021 Aug 28]. Available from: https://www.carnitor.com/pdf/Carnitor-Tablets-OS-SF-PI-04.18-OPI.pdf
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Association of Hospital Pharmacy (Thailand)

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ข้อความภายในบทความที่ตีพิมพ์ในวารสารเภสัชกรรมโรงพยาบาลทั้งหมด รวมถึงรูปภาพประกอบ ตาราง เป็นลิขสิทธิ์ของสมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย) การนำเนื้อหา ข้อความหรือข้อคิดเห็น รูปภาพ ตาราง ของบทความไปจัดพิมพ์เผยแพร่ในรูปแบบต่าง ๆ เพื่อใช้ประโยชน์ในเชิงพาณิชย์ ต้องได้รับอนุญาตจากกองบรรณาธิการวารสาร (สมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย)) อย่างเป็นลายลักษณ์อักษร
สมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย) อนุญาตให้สามารถนำไฟล์บทความไปใช้ประโยชน์และเผยแพร่ต่อได้ โดยอยู่ภายใต้เงื่อนไขสัญญาอนุญาตครีเอทีฟคอมมอน (Creative Commons License: CC) โดย ต้องแสดงที่มาจากวารสาร – ไม่ใช้เพื่อการค้า – ห้ามแก้ไขดัดแปลง, Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0)
ข้อความที่ปรากฏในบทความในวารสารเป็นความคิดเห็นส่วนตัวของผู้เขียนแต่ละท่านไม่เกี่ยวข้องกับสมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย) และบุคลากรในสมาคมฯ แต่อย่างใด ความรับผิดชอบองค์ประกอบทั้งหมดของบทความแต่ละเรื่องเป็นของผู้เขียนแต่ละท่าน หากมีความผิดพลาดใด ๆ ผู้เขียนแต่ละท่านจะรับผิดชอบบทความของตนเอง ตลอดจนความรับผิดชอบด้านเนื้อหาและการตรวจร่างบทความเป็นของผู้เขียน ไม่เกี่ยวข้องกับกองบรรณาธิการ


.png)

