ไอคาทิบานท์: ยารักษาโรคบวมใต้ผิวหนังและเยื่อเมือกจากพันธุกรรม
คำสำคัญ:
ไอคาทิบานท์, โรคบวมใต้ผิวหนังและเยื่อเมือกจากพันธุกรรม, แบรดีไคนินบทคัดย่อ
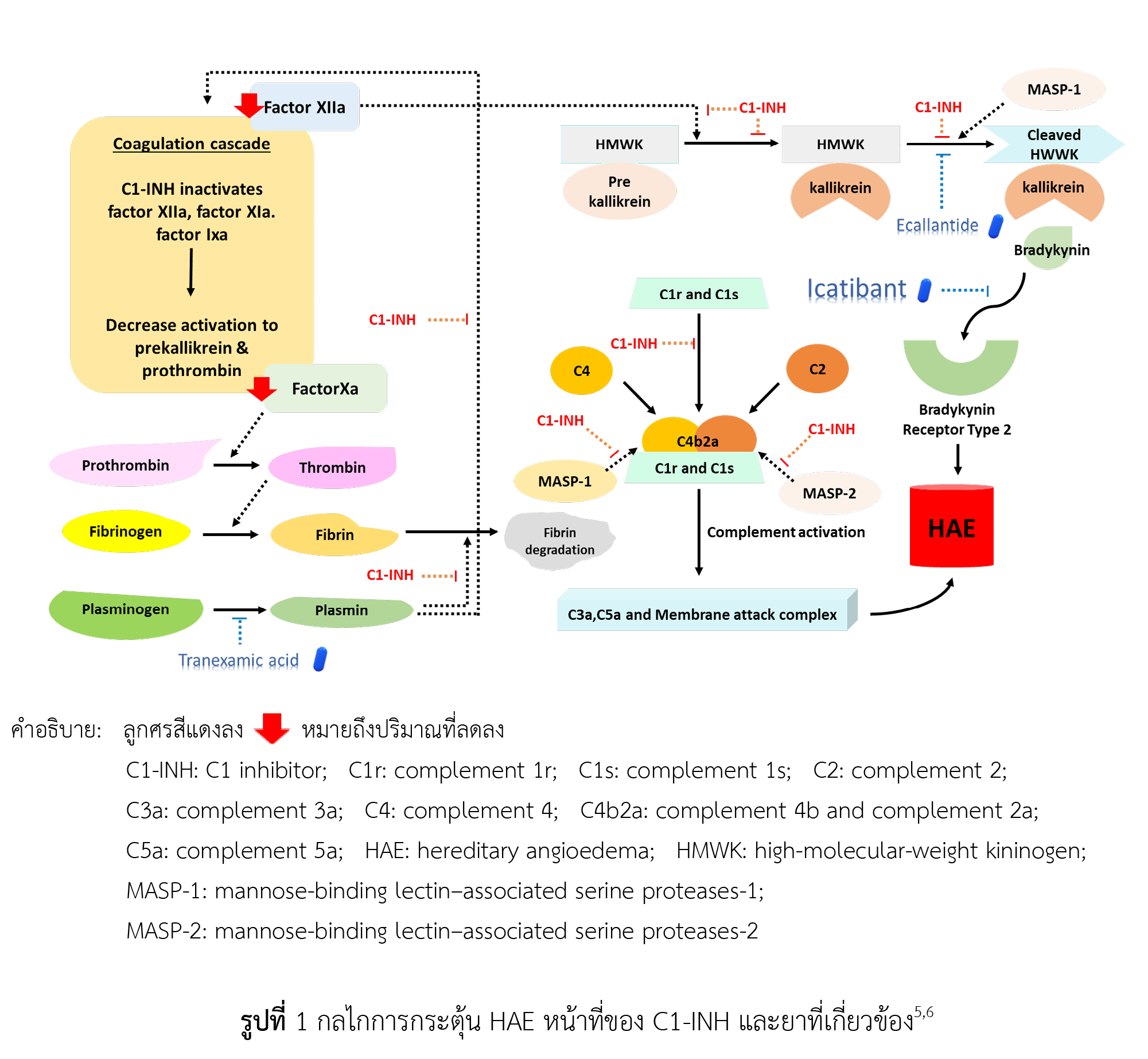
ไอคาทิบานท์เป็นเปปไทด์สังเคราะห์ของกรดอะมิโน 10 ชนิด ที่เลียนแบบโครงสร้างของแบรดีไคนิน มีข้อบ่งใช้ในการรักษาอาการกำเริบจากโรคบวมใต้ผิวหนังและเยื่อเมือกจากพันธุกรรม กลไกการออกฤทธิ์ของยาชนิดนี้คือ แข่งขันกับแบรดีไคนินในการจับตัวรับแบรดีไคนินประเภทที่ 2 ทำให้การขยายตัวของหลอดเลือดและการซึมผ่านของหลอดเลือดฝอยลดลง จากการทบทวนวรรณกรรมพบว่า ไอคาทิบานท์มีประสิทธิผลในการลดความรุนแรงของโรคได้ดีกว่ากลุ่มควบคุมอย่างมีนัยสำคัญทางสถิติ ไอคาทิบานท์จึงถูกแนะนำในแนวเวชปฏิบัติให้ใช้ในการรักษาอาการกำเริบจากโรคบวมใต้ผิวหนังและเยื่อเมือกจากพันธุกรรม นอกจากนี้ ยังมีการนำไอคาทิบานท์มาใช้รักษาโรคบวมใต้ผิวหนังและเยื่อเมือกที่เกิดจากยาในกลุ่ม angiotensin-converting enzyme inhibitors อย่างไรก็ตาม ไอคาทิบานท์กลับมีประสิทธิผลไม่แตกต่างจากกลุ่มควบคุมอย่างมีนัยสำคัญทางสถิติ สำหรับอาการไม่พึงประสงค์ที่พบบ่อย คือ อาการบริเวณที่ฉีด ได้แก่ อาการบวม แดง ปวด ซึ่งส่วนใหญ่ไม่รุนแรงและหายเองได้ โดยสรุปคือไอคาทิบานท์เป็นหนึ่งในตัวยาที่สำคัญที่ใช้รักษาโรคบวมใต้ผิวหนังและเยื่อเมือกจากพันธุกรรม ไม่เพียงแต่ช่วยเพิ่มคุณภาพชีวิตแต่ยังสามารถลดอัตราการตายจากโรคได้
เอกสารอ้างอิง
Maurer M, Magerl M, Betschel S, Aberer W, Ansotegui IJ, Aygören-Pürsün E, et al. The international WAO/EAACI guideline for the management of hereditary angioedema-The 2021 revision and update. Allergy 2022;77(7):1961-90.
Ghazi A, Grant JA. Hereditary angioedema: Epidemiology, management, and role of icatibant. Biologics. 2013;7:103-13.
Busse PJ, Christiansen SC, Riedl MA, Banerji A, Bernstein JA, Castaldo AJ, et al. US HAEA Medical Advisory Board 2020 guidelines for the management of hereditary angioedema. J Allergy Clin Immunol Pract. 2021;9(1):132-50.e3. doi: 10.1016/j.jaip.2020.08.046.
Minafra FG, Gonçalves TR, Alves TM, Pinto JA. The mortality from hereditary angioedema worldwide: a review of the real-world data literature. Clin Rev Allergy Immunol. 2022;62(1):232-9.
Busse PJ, Christiansen SC. Hereditary angioedema. N Engl J Med. 2020;382(12):1136-48.
Morgan BP. Hereditary angioedema--therapies old and new. N Engl J Med. 2010;363(6):581-3.
Firazyr® (icatibant) [product monograph]. Toronto, Ontario, Canada: Takeda Canada Inc; 2020.
Farkas H, Reshef A, Aberer W, Caballero T, McCarthy L, Hao J, et al. Treatment effect and safety of icatibant in pediatric patients with hereditary angioedema. J Allergy Clin Immunol Pract. 2017;5(6):1671-8.e2. doi: 10.1016/j.jaip.2017.04.010.
Gainer JV, Morrow JD, Loveland A, King DJ, Brown NJ. Effect of bradykinin-receptor blockade on the response to angiotensin-converting-enzyme inhibitor in normotensive and hypertensive subjects. N Engl J Med. 1998;339(18):1285-92.
Cicardi M, Banerji A, Bracho F, Malbrán A, Rosenkranz B, Riedl M, et al. Icatibant, a new bradykinin-receptor antagonist, in hereditary angioedema. N Engl J Med. 2010;363(6):532-41.
Lumry WR, Li HH, Levy RJ, Potter PC, Farkas H, Moldovan D, et al. Randomized placebo-controlled trial of the bradykinin B₂ receptor antagonist icatibant for the treatment of acute attacks of hereditary angioedema: The FAST-3 trial. Ann Allergy Asthma Immunol. 2011;107(6):529-37.
Baş M, Greve J, Stelter K, Havel M, Strassen U, Rotter N, et al. A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med. 2015;372(5):418-25.
Sinert R, Levy P, Bernstein JA, Body R, Sivilotti MLA, Moellman J, et al. Randomized trial of icatibant for angiotensin-converting enzyme inhibitor-induced upper airway angioedema. J Allergy Clin Immunol Pract. 2017;5(5):1402-9.e3. doi: 10.1016/j.jaip.2017.03.003.
Straka BT, Ramirez CE, Byrd JB, Stone E, Woodard-Grice A, Nian H, et al. Effect of bradykinin receptor antagonism on ACE inhibitor-associated angioedema. J Allergy Clin Immunol. 2017;140(1):242-8.e2. doi: 10.1016/j.jaci.2016.09.051.
Jeon J, Lee YJ, Lee SY. Effect of icatibant on angiotensin-converting enzyme inhibitor-induced angioedema: a meta-analysis of randomized controlled trials. J Clin Pharm Ther. 2019;44(5):685-92.
Aberer W, Maurer M, Reshef A, Longhurst H, Kivity S, Bygum A, et al. Open-label, multicenter study of self-administered icatibant for attacks of hereditary angioedema. Allergy. 2014;69(3):305-14.
Hernández Fernandez de Rojas D, Ibañez E, Longhurst H, Maurer M, Fabien V, Aberer W, et al. Treatment of HAE attacks in the icatibant outcome survey: an analysis of icatibant self-administration versus administration by health care professionals. Int Arch Allergy Immunol. 2015;167(1):21-8.
ดาวน์โหลด
เผยแพร่แล้ว
รูปแบบการอ้างอิง
ฉบับ
ประเภทบทความ
สัญญาอนุญาต
ลิขสิทธิ์ (c) 2022 สมาคมเภสัชกรรมโรงพยาบาล(ประเทศไทย)

อนุญาตภายใต้เงื่อนไข Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ข้อความภายในบทความที่ตีพิมพ์ในวารสารเภสัชกรรมโรงพยาบาลทั้งหมด รวมถึงรูปภาพประกอบ ตาราง เป็นลิขสิทธิ์ของสมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย) การนำเนื้อหา ข้อความหรือข้อคิดเห็น รูปภาพ ตาราง ของบทความไปจัดพิมพ์เผยแพร่ในรูปแบบต่าง ๆ เพื่อใช้ประโยชน์ในเชิงพาณิชย์ ต้องได้รับอนุญาตจากกองบรรณาธิการวารสาร (สมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย)) อย่างเป็นลายลักษณ์อักษร
สมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย) อนุญาตให้สามารถนำไฟล์บทความไปใช้ประโยชน์และเผยแพร่ต่อได้ โดยอยู่ภายใต้เงื่อนไขสัญญาอนุญาตครีเอทีฟคอมมอน (Creative Commons License: CC) โดย ต้องแสดงที่มาจากวารสาร – ไม่ใช้เพื่อการค้า – ห้ามแก้ไขดัดแปลง, Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0)
ข้อความที่ปรากฏในบทความในวารสารเป็นความคิดเห็นส่วนตัวของผู้เขียนแต่ละท่านไม่เกี่ยวข้องกับสมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย) และบุคลากรในสมาคมฯ แต่อย่างใด ความรับผิดชอบองค์ประกอบทั้งหมดของบทความแต่ละเรื่องเป็นของผู้เขียนแต่ละท่าน หากมีความผิดพลาดใด ๆ ผู้เขียนแต่ละท่านจะรับผิดชอบบทความของตนเอง ตลอดจนความรับผิดชอบด้านเนื้อหาและการตรวจร่างบทความเป็นของผู้เขียน ไม่เกี่ยวข้องกับกองบรรณาธิการ


.png)

