Rezafungin: A New Approach for Treatment of Candidemia and Invasive Candidiasis
Keywords:
rezafungin, candidemia, invasive candidiasisAbstract
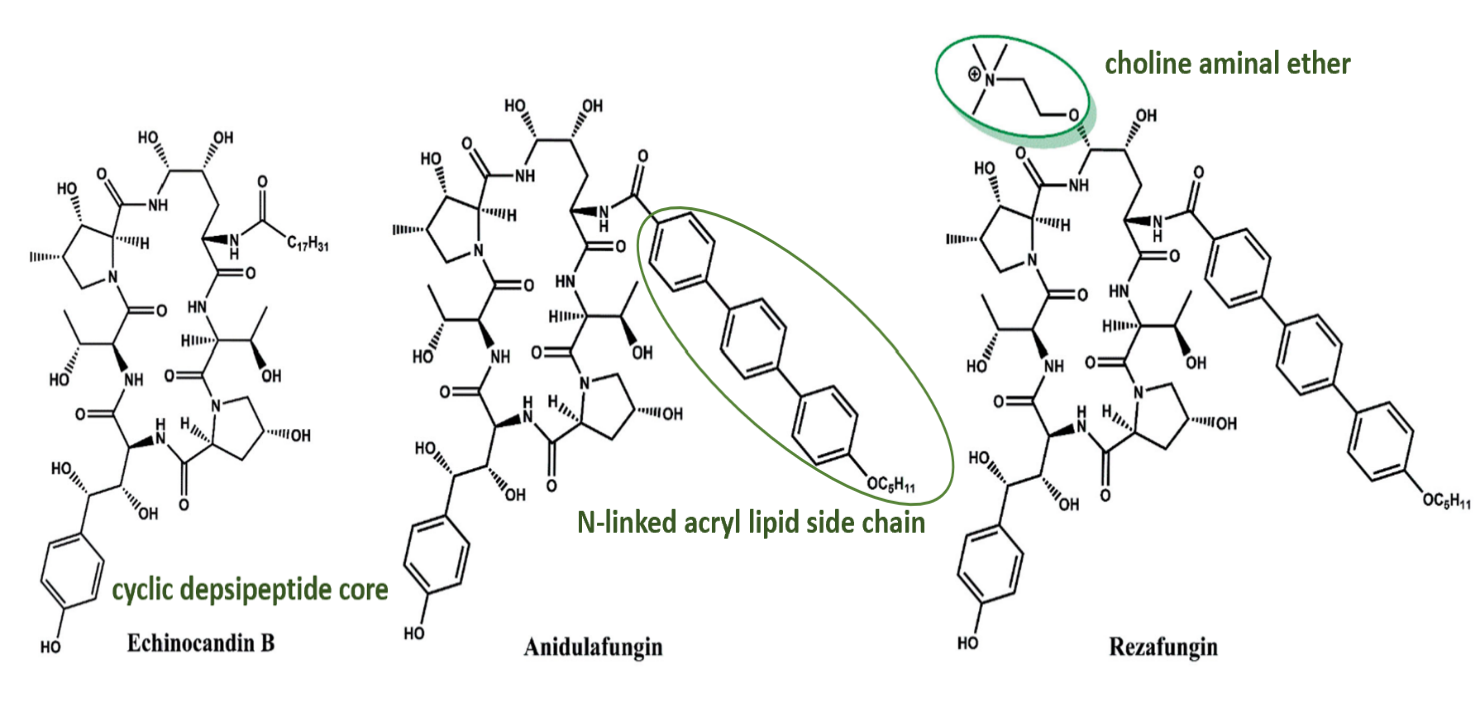
Rezafungin is a second-generation echinocandin, following in the footsteps of anidulafungin, micafungin, and caspofungin. It works by inhibiting 1,3-β-D-glucan synthase, an enzyme involved in the synthesis of fungal cell walls. Administered as a once-weekly intravenous injection, it received approval from the United States Food and Drug Administration on March 22, 2017. Rezafungin is indicated for the treatment of candidemia and invasive candidiasis in patients aged 18 years and older who have limited or no alternative treatment options. Additionally, Rezafungin has been developed for the prevention of fungal infections in patients undergoing bloodstream and bone marrow transplants. Systematic reviews have demonstrated that its long half-life allows for weekly administration. Rezafungin is also safer to use than first-generation echinocandins, as it has lower hepatotoxicity.
References
McCarty TP, White CM, Pappas PG. Candidemia and Invasive Candidiasis. Infect Dis Clin North Am. 2021;35(2):389-413. doi: 10.1016/j.idc.2021.03.007.
de Oliveira Santos GC, Vasconcelos CC, Lopes AJO, de Sousa Cartágenes MDS, Filho AKDB, do Nascimento FRF, et al. Candida infections and therapeutic strategies: mechanisms of action for traditional and alternative agents. Front Microbiol. 2018;9:1351. doi: 10.3389/fmicb.2018.01351.
European Medicines Agency (EMA). Public summary of opinion on orphan designation: rezafungin acetate for the treatment of invasive candidiasis. EU designation No. EU/3/20/2385 [Internet]. Amsterdam: EMA; 2021 [cited 2023 Jul 18]. Available from: https://www.ema.europa.eu/en/documents/orphan-designation/eu/3/20/2385-public-summary-opinion-orphan-designation-rezafungin-acetate-treatment-invasive-candidiasis_en.pdf
Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1-50. doi: 10.1093/cid/civ933.
Tan BH, Chakrabarti A, Li RY, Patel AK, Watcharananan SP, Liu Z, et al. Incidence and species distribution of candidaemia in Asia: a laboratory-based surveillance study. Clin Microbiol Infect. 2015;21(10):946-53. doi: 10.1016/j.cmi.2015.06.010.
Syed YY. Rezafungin: first approval. Drugs. 2023;83(9):833-40. doi: 10.1007/s40265-023-01891-8.
Melinta Therapeutics LLC. REZZAYO™ (rezafungin for injection), for intravenous use [Internet]. Lincolnshire (IL): Melinta Therapeutics LLC; 2023. [cited 2023 Jul 18]. Available from: https://www.rezzayo.com/wp-content/pdfs/REZZAYO%20(rezafungin%20for%20injection)%20Package%20Insert.pdf
Szymanski M, Chmielewska S, Czyzewska U, Malinowska M, Tylicki A. Echinocandins - structure, mechanism of action and use in antifungal therapy. J Enzyme Inhib Med Chem. 2022;37(1):876-94. doi: 10.1080/14756366.2022.2050224.
Zhao Y, Perlin DS. Review of the novel echinocandin antifungal rezafungin: animal studies and clinical data. J Fungi (Basel). 2020;6(4):192. doi: 10.3390/jof6040192.
McKeny PT, Nessel TA, Zito PM. Antifungal antibiotics. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 [cited 2023 Jul 18]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538168/
Robbins N, Wright GD, Cowen LE. Antifungal drugs: the current armamentarium and development of new agents. Microbiol Spectr. 2016;4(5). doi: 10.1128/microbiolspec.FUNK-0002-2016.
Merative Corporation. DRUGDEX [data on the internet]. Ann Arbor (MI): Micromedex, Merative Corporation; 2023 [cited 2024 May 10]. Available from: https://www.micromedexsolutions.com/micromedex2/librarian/CS/C56967/ND_PR/evidencexpert/ND_P/evidencexpert/ (Subscription required)
Lepak AJ, Andes DR. Antifungal pharmacokinetics and pharmacodynamics. Cold Spring Harb Perspect Med. 2014;5(5):a019653. doi: 10.1101/cshperspect.a019653.
Bellmann R, Smuszkiewicz P. Pharmacokinetics of antifungal drugs: practical implications for optimized treatment of patients. Infection. 2017;45(6):737-79. doi: 10.1007/s15010-017-1042-z.
Thompson GR 3rd, Soriano A, Skoutelis A, Vazquez JA, Honore PM, Horcajada JP, et al. Rezafungin versus caspofungin in a phase 2, randomized, double-blind study for the treatment of candidemia and invasive candidiasis: the STRIVE trial. Clin Infect Dis. 2021;73(11):e3647-55. doi: 10.1093/cid/ciaa1380.
Thompson GR 3rd, Soriano A, Cornely OA, Kullberg BJ, Kollef M, Vazquez J, et al. Rezafungin versus caspofungin for treatment of candidaemia and invasive candidiasis (ReSTORE): a multicentre, double-blind, double-dummy, randomised phase 3 trial. Lancet. 2023;401(10370):49-59. doi: 10.1016/S0140-6736(22)02324-8.
Thompson GR 3rd, Soriano A, Honore PM, Bassetti M, Cornely OA, Kollef M, et al. Efficacy and safety of rezafungin and caspofungin in candidaemia and invasive candidiasis: pooled data from two prospective randomised controlled trials. Lancet Infect Dis. 2024;24(3):319-28. doi: 10.1016/S1473-3099(23)00551-0.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Association of Hospital Pharmacy (Thailand)

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ข้อความภายในบทความที่ตีพิมพ์ในวารสารเภสัชกรรมโรงพยาบาลทั้งหมด รวมถึงรูปภาพประกอบ ตาราง เป็นลิขสิทธิ์ของสมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย) การนำเนื้อหา ข้อความหรือข้อคิดเห็น รูปภาพ ตาราง ของบทความไปจัดพิมพ์เผยแพร่ในรูปแบบต่าง ๆ เพื่อใช้ประโยชน์ในเชิงพาณิชย์ ต้องได้รับอนุญาตจากกองบรรณาธิการวารสาร (สมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย)) อย่างเป็นลายลักษณ์อักษร
สมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย) อนุญาตให้สามารถนำไฟล์บทความไปใช้ประโยชน์และเผยแพร่ต่อได้ โดยอยู่ภายใต้เงื่อนไขสัญญาอนุญาตครีเอทีฟคอมมอน (Creative Commons License: CC) โดย ต้องแสดงที่มาจากวารสาร – ไม่ใช้เพื่อการค้า – ห้ามแก้ไขดัดแปลง, Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0)
ข้อความที่ปรากฏในบทความในวารสารเป็นความคิดเห็นส่วนตัวของผู้เขียนแต่ละท่านไม่เกี่ยวข้องกับสมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย) และบุคลากรในสมาคมฯ แต่อย่างใด ความรับผิดชอบองค์ประกอบทั้งหมดของบทความแต่ละเรื่องเป็นของผู้เขียนแต่ละท่าน หากมีความผิดพลาดใด ๆ ผู้เขียนแต่ละท่านจะรับผิดชอบบทความของตนเอง ตลอดจนความรับผิดชอบด้านเนื้อหาและการตรวจร่างบทความเป็นของผู้เขียน ไม่เกี่ยวข้องกับกองบรรณาธิการ


.png)

