เบนราลิซูแมบ ยารักษาโรคหืดรุนแรงชนิดใหม่
คำสำคัญ:
เบนราลิซูแมบ, โรคหืด, แอนตี้อีโอซิโนฟิลล์บทคัดย่อ
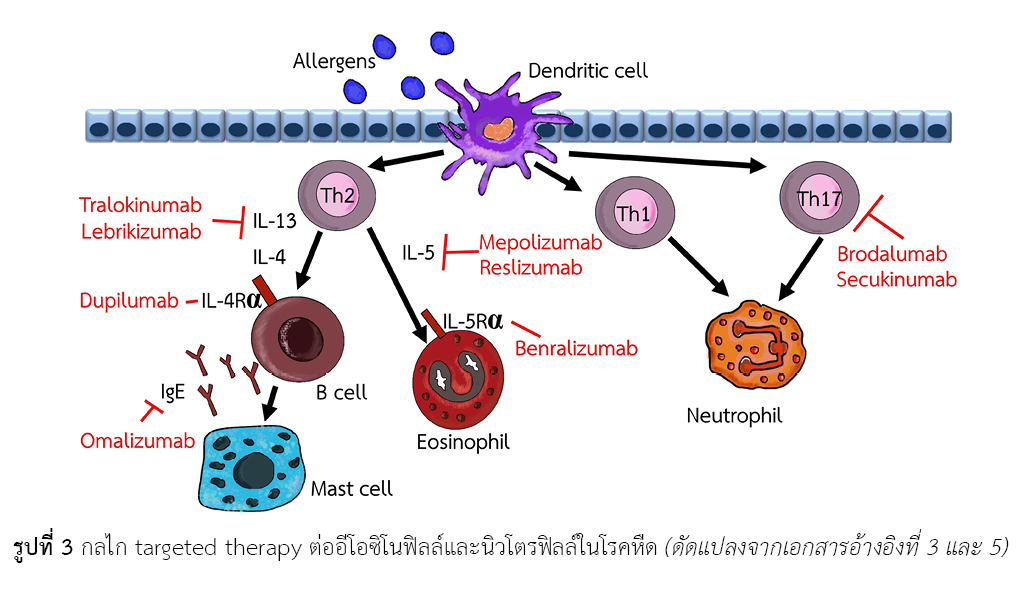
เบนราลิซูแมบ เป็นยาในกลุ่ม monoclonal antibody ที่ออกฤทธิ์จับแบบเฉพาะเจาะจงกับ alpha subunit ของ interleukin-5 (IL-5) receptor ของมนุษย์ ทำให้ขัดขวางการจับของ IL-5 กับตัวรับ ตามมาด้วยการขัดขวางการแบ่งตัวและการเติบโตของอีโอซิโนฟิลล์และแบโซฟิลล์ เป็นผลให้มีจำนวนอีโอซิโนฟิลล์และแบโซฟิลล์ในเลือดลดลงผ่านกลไก antibody-dependent cell-mediated cytotoxicity ยามีข้อบ่งใช้ร่วมกับการรักษาเดิมในผู้ป่วยผู้ใหญ่ที่เป็นโรคหืดรุนแรงที่เกิดจากการอักเสบชนิดอีโอซิโนฟิลล์ที่ไม่สามารถควบคุมอาการได้แม้จะใช้ยาคอร์ติโคสเตียรอยด์ชนิดพ่นสูดขนาดสูงผสมกับยาขยายหลอดลมที่ออกฤทธิ์ยาวแล้วก็ตาม จากการรวบรวมงานวิจัยที่เปรียบเทียบประสิทธิภาพพบว่าการใช้เบนราลิซูแมบ สามารถลดอัตราการเกิดโรคหืดกำเริบใน 1 ปีได้มากกว่ายาหลอกอย่างมีนัยสำคัญทางสถิติ ช่วยเพิ่มสมรรถภาพปอด (แสดงด้วยค่า FEV1) มีคุณภาพชีวิตที่ดี สามารถควบคุมอาการของโรคหืด ลดขนาดการใช้ยาคอร์ติโคสเตียรอยด์ชนิดรับประทาน และสามารถลดระดับอีโอซิโนฟิลล์ในเลือดได้ อย่างไรก็ตาม อาการไม่พึงประสงค์ที่พบบ่อยคือ เยื่อจมูกและลำคออักเสบเฉียบพลัน อาการหอบหืดที่แย่ลง หลอดลมอักเสบ มีปฏิกิริยาบริเวณที่ฉีดยา การติดเชื้อทางเดินหายใจส่วนบน เป็นต้น หากเปรียบเทียบด้านความคุ้มค่าจากการวิเคราะห์ต้นทุน-ประสิทธิผล พบว่า เบนราลิซูแมบ มีความคุ้มค่าในการใช้ร่วมกับการรักษาเดิมในผู้ป่วยผู้ใหญ่ที่เป็นโรคหืดรุนแรง ที่เกิดจากการอักเสบชนิดอีโอซิโนฟิลล์ที่ดื้อต่อการรักษา
เอกสารอ้างอิง
สมาคมสภาองค์กรโรคหืดแห่งประเทศไทย. แนวทางการวินิจฉัยและรักษาหืดในประเทศไทยสำหรับผู้ใหญ่ พ.ศ. 2562. พิมพ์ครั้งที่ 1. กรุงเทพ ฯ: บริษัท บียอนด์ เอ็นเทอร์ไพรซ์ จำกัด; 2561.
GINA. Global strategy for asthma management and prevention 2021 [Internet]. Fontana, USA [cited 2021 Jul 4]. Available from: https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf.
ธีระศักดิ์ แก้วอมตวงศ์. การรักษามุ่งเป้าสำหรับโรคหืดรุนแรง. พิมพ์ครั้งที่ 1. กรุงเทพฯ: คณะแพทยศาสตร์โรงพยาบาลรามาธิบดี มหาวิทยาลัยมหิดล; 2560.
Bel EH, Brinke AT. New anti-eosinophil drugs for asthma and COPD: Targeting the trait. Chest. 2017;152(6):1276-82.
Bice JB, Leechawengwongs E, Montanaro A. Biologic targeted therapy in allergic asthma. Ann Allergy Asthma Immunol. 2014;112(2):108-15.
Fasenra® [package insert]. Bangkok (Thailand): AstraZeneca; 2019.
Drugbank [Internet]. Alberta Canada; c2015. Drug information: Benralizumab; [updated 2021 Jul 8; cited 2021 Jul 12]. Available from: https://go.drugbank.com/drugs/DB12023.
Ghazi A, Trikha A, Calhoun WJ. Benralizumab – a humanized mAb to IL-5Rα with enhanced antibody-dependent cell-mediated cytotoxicity – a novel approach for the treatment of asthma. Expert Opin Biol Ther. 2012;12(1):113–8.
Sista U, Ren Y, Yu J, Marathe A, Ji P. Clinical pharmacology and biopharmaceutics review, benralizumab [Internet]. 2016 [cited 2021 Jul 12]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/761070Orig1s000ClinPharmR.pdf.
Bleecker RE, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): A randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115-27.
FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128-41.
Tian BP, Zhang GS, Lou J, Zhou HB, Cui W. Efficacy and safety of benralizumab for eosinophilic asthma: A systematic review and meta-analysis of randomized controlled trials. J Asthma. 2018;55(9):956-65.
Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. Oral glucocorticoid–sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448-58.
Galo AP, Ruiz AJG, Abitbol ACL, Olveira C, Ruiz FR, Soler NGA, et al. Real-life cost-effectiveness of benralizumab in patients with severe asthma. Respir Res. 2021;22(163):1-14.
Agache I, Rocha C, Beltran J, Song Y, Posso M, Solà I, et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab and omalizumab) for severe allergic asthma: A systematic review for the EAACI Guidelines – recommendations on the use of biologicals in severe asthma. Allergy. 2020;75:1043–57.
ดาวน์โหลด
เผยแพร่แล้ว
รูปแบบการอ้างอิง
ฉบับ
ประเภทบทความ
สัญญาอนุญาต
ลิขสิทธิ์ (c) 2022 สมาคมเภสัชกรรมโรงพยาบาล(ประเทศไทย)

อนุญาตภายใต้เงื่อนไข Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ข้อความภายในบทความที่ตีพิมพ์ในวารสารเภสัชกรรมโรงพยาบาลทั้งหมด รวมถึงรูปภาพประกอบ ตาราง เป็นลิขสิทธิ์ของสมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย) การนำเนื้อหา ข้อความหรือข้อคิดเห็น รูปภาพ ตาราง ของบทความไปจัดพิมพ์เผยแพร่ในรูปแบบต่าง ๆ เพื่อใช้ประโยชน์ในเชิงพาณิชย์ ต้องได้รับอนุญาตจากกองบรรณาธิการวารสาร (สมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย)) อย่างเป็นลายลักษณ์อักษร
สมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย) อนุญาตให้สามารถนำไฟล์บทความไปใช้ประโยชน์และเผยแพร่ต่อได้ โดยอยู่ภายใต้เงื่อนไขสัญญาอนุญาตครีเอทีฟคอมมอน (Creative Commons License: CC) โดย ต้องแสดงที่มาจากวารสาร – ไม่ใช้เพื่อการค้า – ห้ามแก้ไขดัดแปลง, Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0)
ข้อความที่ปรากฏในบทความในวารสารเป็นความคิดเห็นส่วนตัวของผู้เขียนแต่ละท่านไม่เกี่ยวข้องกับสมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย) และบุคลากรในสมาคมฯ แต่อย่างใด ความรับผิดชอบองค์ประกอบทั้งหมดของบทความแต่ละเรื่องเป็นของผู้เขียนแต่ละท่าน หากมีความผิดพลาดใด ๆ ผู้เขียนแต่ละท่านจะรับผิดชอบบทความของตนเอง ตลอดจนความรับผิดชอบด้านเนื้อหาและการตรวจร่างบทความเป็นของผู้เขียน ไม่เกี่ยวข้องกับกองบรรณาธิการ


.png)

