กรณีศึกษาภาวะถอนยาจากเฟนทานิลในผู้ป่วยวิกฤตเด็ก
คำสำคัญ:
เฟนทานิล, อาการถอนยา, อาการไม่พึงประสงค์จากยา, ผู้ป่วยวิกฤต, ผู้ป่วยเด็กบทคัดย่อ
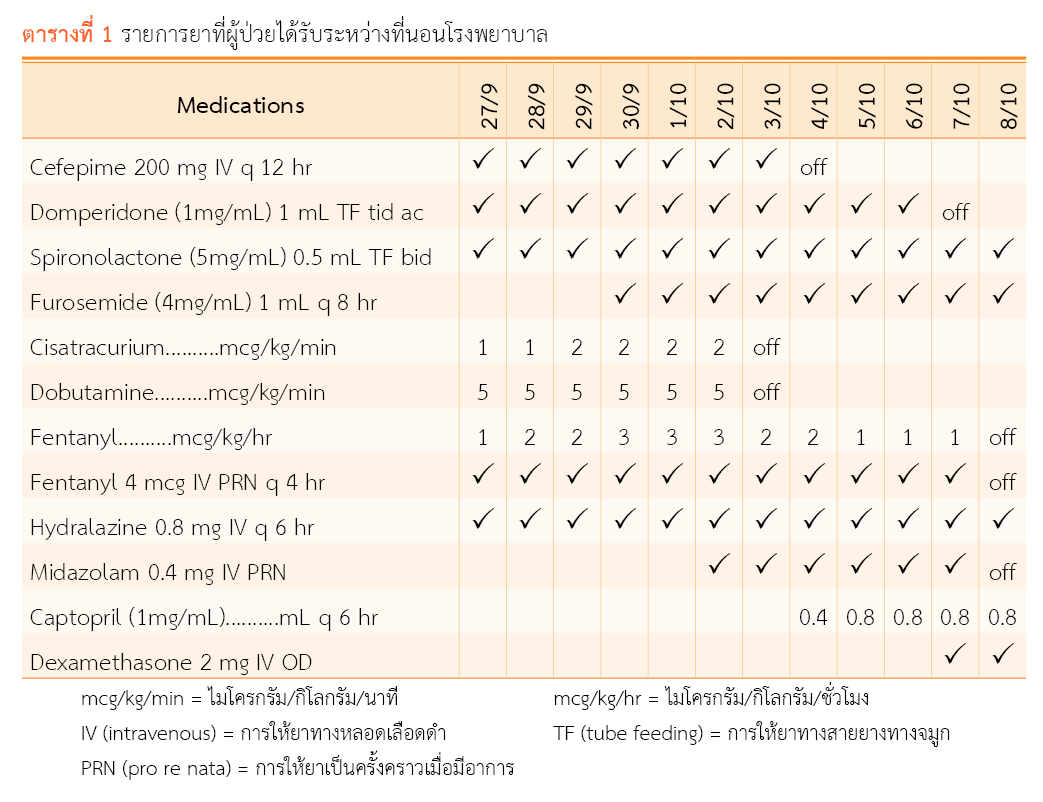
ผู้ป่วยเด็กในหอผู้ป่วยเวชบำบัดวิกฤตมักจะมีความเจ็บปวดจากสภาวะโรคและการทำหัตถการ รวมถึงในบางรายมีการใส่ท่อช่วยหายใจ จึงจำเป็นต้องได้รับยาระงับปวดและยาสงบระงับ โดยยาที่มีการใช้บ่อย คือ เฟนทานิล อย่างไรก็ตามพบว่าการใช้เฟนทานิลขนาดสูงและระยะเวลานานแล้วหยุดทันทีทำให้เกิดอาการถอนยาตามมาได้ ซึ่งอาการดังกล่าวสามารถป้องกันได้โดยการให้เฟนทานิลในขนาดที่เหมาะสม หยุดยาเมื่อหมดข้อบ่งใช้ แต่หากได้รับเฟนทานิลเกินกว่า 5 วันหรือขนาดยาสะสมเกิน 0.48 มิลลิกรัม/กิโลกรัม ควรค่อยๆ ลดขนาดยาและพิจารณาให้ยาในกลุ่มโอปิออยด์อื่นที่มีค่าครึ่งชีวิตยาวกว่า เช่น เมททาโดน หรือมอร์ฟีนรูปแบบรับประทานทดแทน บทความนี้เป็นกรณีศึกษา ผู้ป่วยเด็กไทยเพศชาย อายุ 1 เดือน เป็นโรคหัวใจพิการแต่กำเนิด ได้รับเฟนทานิลขนาด 1 – 3 ไมโครกรัม/กิโลกรัม/ชั่วโมง เป็นเวลานาน 11 วัน เมื่อหยุดเฟนทานิลผู้ป่วยมีอาการกระวนกระวาย อาเจียน หายใจเร็ว และเหงื่อแตก ทำการประเมิน withdrawal assessment tool-1 ได้ 4 คะแนน ซึ่งเข้าได้กับอาการถอนยากลุ่มโอปิออยด์ จึงให้มอร์ฟีนรูปแบบรับประทานทดแทนเฟนทานิล และลดมอร์ฟีนอย่างช้าๆ จนสามารถหยุดยาได้ โดยใช้เวลาในการรักษาอาการถอนยา 12 วัน
จากกรณีศึกษาแสดงให้เห็นว่าอาการถอนยาเป็นอาการที่สามารถป้องกันและรักษาได้ เภสัชกรมีบทบาทในการช่วยประเมินความเสี่ยงในการเกิดภาวะถอนยา รวมทั้งให้คำแนะนำในการเลือกยาทดแทนแก่แพทย์ และคำนวณขนาดยาทดแทนที่เหมาะสมในผู้ป่วยแต่ละราย
เอกสารอ้างอิง
Ramos-Matos CF, Bistas KG, Lopez-Ojeda W. Fentanyl [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 [cited 2023 Nov 4]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459275
Ziesenitz VC, Vaughns JD, Koch G, Mikus G, van den Anker JN. Pharmacokinetics of fentanyl and its derivatives in children: a comprehensive review. Clin Pharmacokinet. 2018;57(2):125-49. doi: 10.1007/s40262-017-0569-6.
Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, et al. Opioid complications and side effects. Pain Physician. 2008;11(2 Suppl):S105-20. PMID: 18443635.
Fernández-Carrión F, Gaboli M, González-Celador R, Gómez de Quero-Masía P, Fernández-de Miguel S, Murga-Herrera V, et al. Withdrawal syndrome in the pediatric intensive care unit. Incidence and risk factors. Med Intensiva. 2013;37(2):67-74. doi: 10.1016/j.medin.2012.02.009.
Tammen AJ, Brescia D, Jonas D, Hodges JL, Keith P. Fentanyl-induced rigid chest syndrome in critically ill patients. J Intensive Care Med. 2023;38(2):196-201. doi: 10.1177/08850666221115635.
Habib E, Almakadma AH, Albarazi M, Jaimon S, Almehizia R, Al Wadai A, et al. Iatrogenic withdrawal syndrome in the pediatric cardiac intensive care unit: incidence, risk factors and outcome. J Saudi Heart Assoc. 2021;33(4):251-60. doi: 10.37616/2212-5043.1268.
Kristie RO, Anabel PR, Carlos LO, Samuel PR, Gabriel JA, Ricardo N, et al. Withdrawal syndrome incidence and risk factors in pediatric intensive care unit. Crit Care Med. 2016;44(12):269. doi: 10.1097/01.ccm.0000509450.41951.46.
Franck LS, Scoppettuolo LA, Wypij D, Curley MAQ. Validity and generalizability of the withdrawal assessment tool-1 (WAT-1) for monitoring iatrogenic withdrawal syndrome in pediatric patients. Pain. 2012;153(1):142-8. doi: 10.1016/j.pain.2011.10.003.
Tiacharoen D, Lertbunrian R, Veawpanich J, Suppalarkbunlue N, Anantasit N. Protocolized sedative weaning vs usual care in pediatric critically ill patients: a pilot randomized controlled trial. Indian J Crit Care Med. 2020;24(6):451-8. doi: 10.5005/jp-journals-10071-23465.
Best KM, Asaro LA, Franck LS, Wypij D, Curley MA; Randomized evaluation of sedation titration for respiratory failure baseline study investigators. Patterns of sedation weaning in critically ill children recovering from acute respiratory failure. Pediatr Crit Care Med. 2016;17(1):19-29. doi: 10.1097/PCC.0000000000000572.
Thigpen JC, Odle BL, Harirforoosh S. Opioids: a review of pharmacokinetics and pharmacodynamics in neonates, infants, and children. Eur J Drug Metab Pharmacokinet. 2019;44(5):591-609. doi: 10.1007/s13318-019-00552-0.
Schwinghammer AJ, Wilson MD, Hall BA. Corrected QT interval prolongation in hospitalized pediatric patients receiving methadone. Pediatr Crit Care Med. 2018;19(8):e403-8. doi: 10.1097/PCC.0000000000001601.
Von Korff M, Saunders K, Thomas RG, Boudreau D, Campbell C, Merrill J, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24(6):521-7. doi: 10.1097/AJP.0b013e318169d03b.
Fine PG, Portenoy RK; Ad Hoc Expert Panel on Evidence Review and Guidelines for Opioid Rotation. Establishing "best practices" for opioid rotation: conclusions of an expert panel. J Pain Symptom Manage. 2009;38(3):418-25. doi: 10.1016/j.jpainsymman.2009.06.002.
ดาวน์โหลด
เผยแพร่แล้ว
รูปแบบการอ้างอิง
ฉบับ
ประเภทบทความ
สัญญาอนุญาต
ลิขสิทธิ์ (c) 2024 สมาคมเภสัชกรรมโรงพยาบาล(ประเทศไทย)

อนุญาตภายใต้เงื่อนไข Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ข้อความภายในบทความที่ตีพิมพ์ในวารสารเภสัชกรรมโรงพยาบาลทั้งหมด รวมถึงรูปภาพประกอบ ตาราง เป็นลิขสิทธิ์ของสมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย) การนำเนื้อหา ข้อความหรือข้อคิดเห็น รูปภาพ ตาราง ของบทความไปจัดพิมพ์เผยแพร่ในรูปแบบต่าง ๆ เพื่อใช้ประโยชน์ในเชิงพาณิชย์ ต้องได้รับอนุญาตจากกองบรรณาธิการวารสาร (สมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย)) อย่างเป็นลายลักษณ์อักษร
สมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย) อนุญาตให้สามารถนำไฟล์บทความไปใช้ประโยชน์และเผยแพร่ต่อได้ โดยอยู่ภายใต้เงื่อนไขสัญญาอนุญาตครีเอทีฟคอมมอน (Creative Commons License: CC) โดย ต้องแสดงที่มาจากวารสาร – ไม่ใช้เพื่อการค้า – ห้ามแก้ไขดัดแปลง, Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0)
ข้อความที่ปรากฏในบทความในวารสารเป็นความคิดเห็นส่วนตัวของผู้เขียนแต่ละท่านไม่เกี่ยวข้องกับสมาคมเภสัชกรรมโรงพยาบาล (ประเทศไทย) และบุคลากรในสมาคมฯ แต่อย่างใด ความรับผิดชอบองค์ประกอบทั้งหมดของบทความแต่ละเรื่องเป็นของผู้เขียนแต่ละท่าน หากมีความผิดพลาดใด ๆ ผู้เขียนแต่ละท่านจะรับผิดชอบบทความของตนเอง ตลอดจนความรับผิดชอบด้านเนื้อหาและการตรวจร่างบทความเป็นของผู้เขียน ไม่เกี่ยวข้องกับกองบรรณาธิการ


.png)

